Nucleosides, Nucleotides & Nucleic Acids
2003-10-01
Convenient synthesis of 2'-deoxy-2-fluoroadenosine from 2-fluoroadenine.
Song Ye, Martha M Rezende, Wei-Ping Deng, Kenneth L Kirk
Index: Nucleosides Nucleotides Nucleic Acids 22(10) , 1899-905, (2003)
Full Text: HTML
Abstract
A convenient synthesis of 2'-deoxy-2-fluoroadenosine from commercially available 2-fluoroadenine is described. The coupling reaction of silylated 2-fluoroadenine with phenyl 3,5-bis[O-(t-butyldimethylsilyl)]-2-deoxy-1-thio-D-erythro-pentofuranoside gave the corresponding 2-fluoro-2'-deoxyadenosine derivative (alpha/beta = 1:1) in good yield. The alpha- and beta-anomers were separated by chromatography, and then desilylated to give compounds 1a and 1b.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
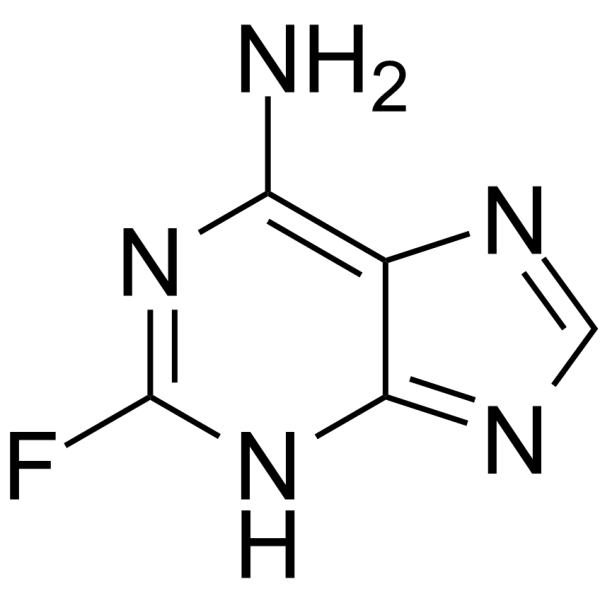 |
TCMDC-124283
CAS:700-49-2 |
C5H4FN5 |
Related Articles:
More...
|
Reduced drug incorporation into DNA and antiapoptosis as the...
2014-01-01 [BMC Cancer 14 , 547, (2014)] |
|
Endothelin-1 promotes survival and chemoresistance in chroni...
2014-01-01 [PLoS ONE 9(6) , e98818, (2014)] |
|
Preparation of a fludarabine intermediate via selective alky...
2006-01-01 [Nucleosides Nucleotides Nucleic Acids 25(7) , 735-45, (2006)] |
|
Metabolism and metabolic actions of 6-methylpurine and 2-flu...
1998-05-15 [Biochem. Pharmacol. 55(10) , 1673-81, (1998)] |
|
Synthesis and biological activity of 2-fluoro adenine and 6-...
2005-01-01 [Nucleosides Nucleotides Nucleic Acids 24 , 881-885, (2005)] |