| Structure | Name/CAS No. | Articles |
|---|---|---|
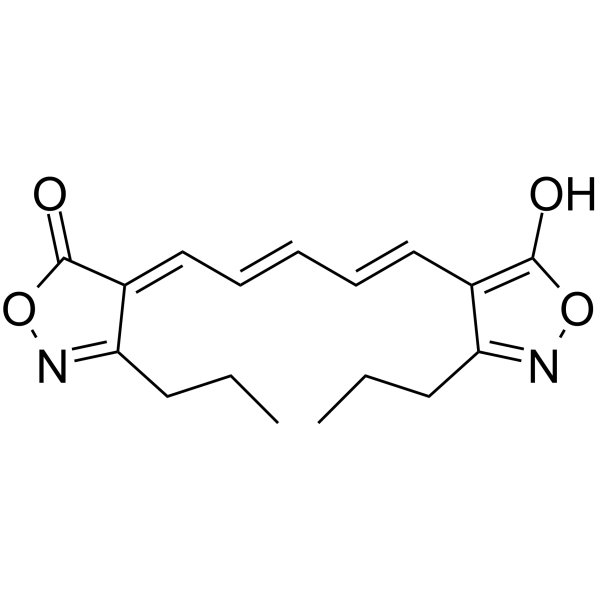 |
Oxonol VI
CAS:64724-75-0 |
|
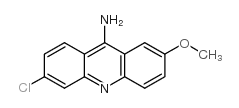 |
9-AMINO-6-CHLORO-2-METHOXYACRIDINE
CAS:3548-09-2 |