| Structure | Name/CAS No. | Articles |
|---|---|---|
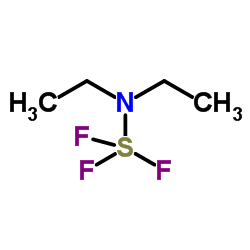 |
Diethylaminosulfur trifluoride
CAS:38078-09-0 |
|
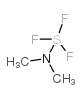 |
Methyl DAST
CAS:3880-03-3 |