Journal of Organic Chemistry
2012-07-20
Ring expansion of cyclic β-amino alcohols induced by diethylaminosulfur trifluoride: synthesis of cyclic amines with a tertiary fluorine at C3.
Bruno Anxionnat, Benoit Robert, Pascal George, Gino Ricci, Marc-Antoine Perrin, Domingo Gomez Pardo, Janine Cossy
Index: J. Org. Chem. 77(14) , 6087-99, (2012)
Full Text: HTML
Abstract
As the replacement of a hydrogen atom by a fluorine atom in a compound can have an important impact on its biological properties, the development of methods allowing the introduction of a fluorine atom is of great importance. The scope and limitations of the ring expansion of cyclic 2-hydroxymethyl amines induced by diethylaminosulfur trifluoride (DAST) to produce cyclic β-fluoro amines was studied as well as the enantioselectivity of the process.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
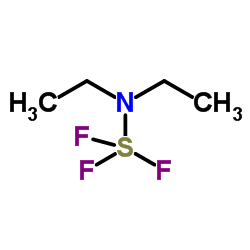 |
Diethylaminosulfur trifluoride
CAS:38078-09-0 |
C4H10F3NS |
Related Articles:
More...
|
Rearrangement of homoallylic alcohols induced by DAST.
2006-05-11 [Org. Lett. 8 , 2091, (2006)] |
|
Fluorinative Beckmann fragmentation: fluorinative alpha-clea...
2000-02-01 [Chem. Pharm. Bull. 48(2) , 220-2, (2000)] |
|
Synthesis of functionalized oxazolines and oxazoles with DAS...
2000-04-20 [Org. Lett. 2(8) , 1165-8, (2000)] |
|
Solid-phase chemical synthesis and in vitro biological evalu...
2012-11-01 [Steroids 77(13) , 1403-18, (2012)] |
|
Stereo- and regio-selectivity of diethylaminosulfur trifluor...
1983-09-16 [Carbohydr. Res. 121 , 51-60, (1983)] |