| Structure | Name/CAS No. | Articles |
|---|---|---|
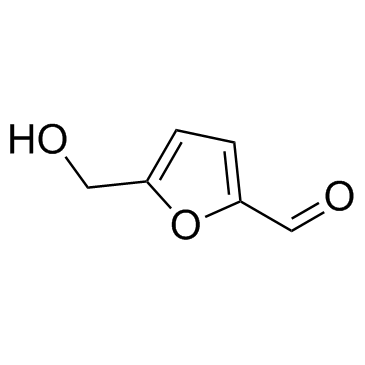 |
5-hydroxymethylfurfural
CAS:67-47-0 |
|
![1H-Pyrido[3,4-b]indole-3-carboxylicacid, 2,3,4,9-tetrahydro-1-methyl Structure](https://image.chemsrc.com/caspic/209/5470-37-1.png) |
1H-Pyrido[3,4-b]indole-3-carboxylicacid, 2,3,4,9-tetrahydro-1-methyl
CAS:5470-37-1 |