| Structure | Name/CAS No. | Articles |
|---|---|---|
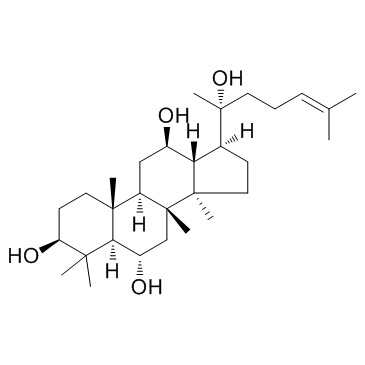 |
Protopanaxatriol
CAS:1453-93-6 |
|
 |
Ginsenoside Rh1
CAS:63223-86-9 |
|
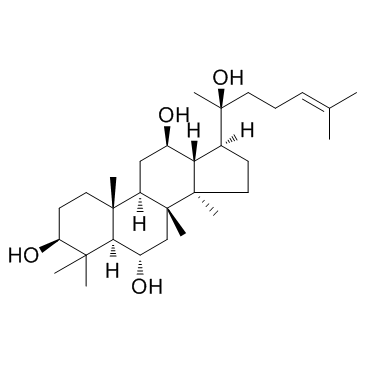 |
(20S)-Protopanaxatriol
CAS:34080-08-5 |