| Structure | Name/CAS No. | Articles |
|---|---|---|
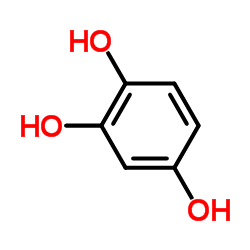 |
1,2,4-Trihydroxybenzene
CAS:533-73-3 |
|
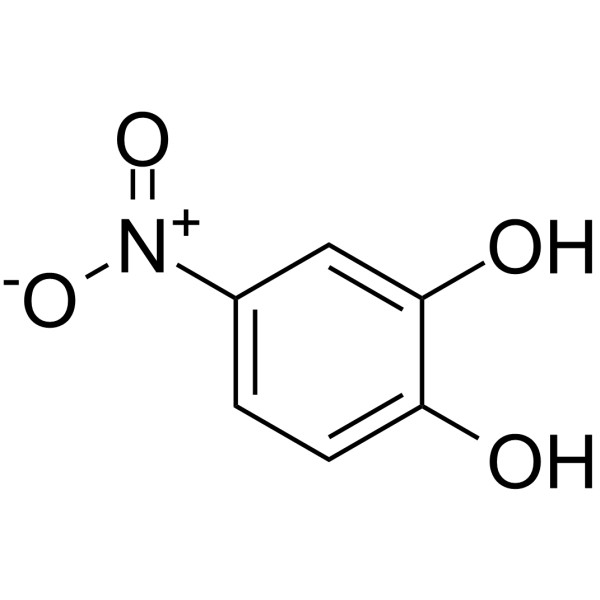 |
4-nitrocatechol
CAS:3316-09-4 |