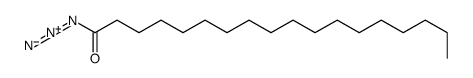
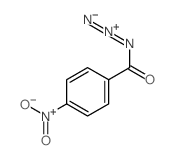
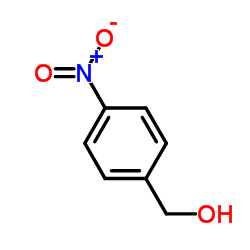
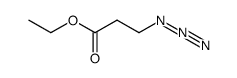
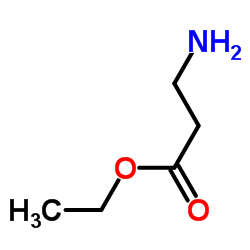
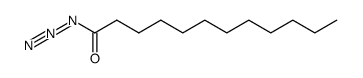
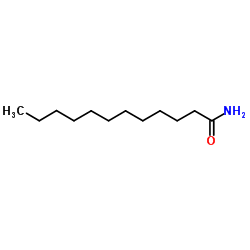
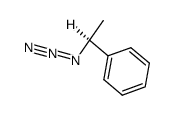
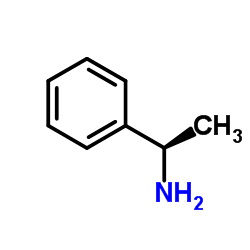
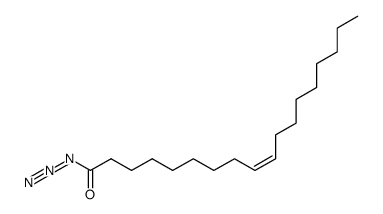
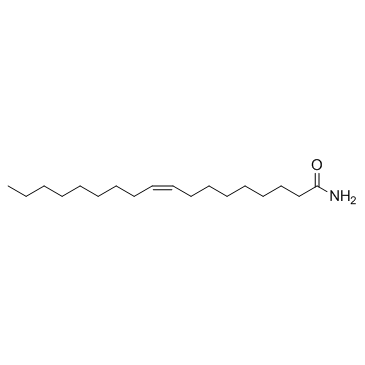
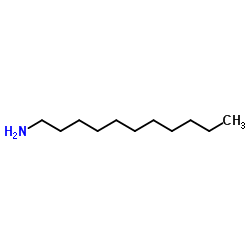
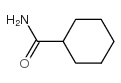
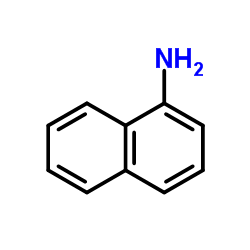
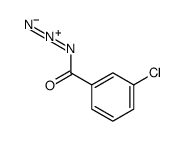
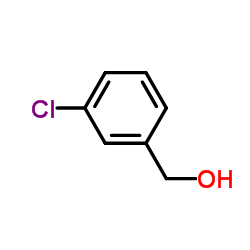
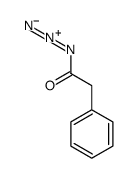
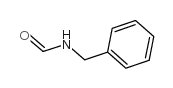
|
~92% |
|
~93% |
|
~85% |
|
~93% |
|
~63% |
|
~85% |
|
~92% |
|
~87% |
|
~87% |
|
~96% |
|
~92% |
|
~86% |