| Structure | Name/CAS No. | Articles |
|---|---|---|
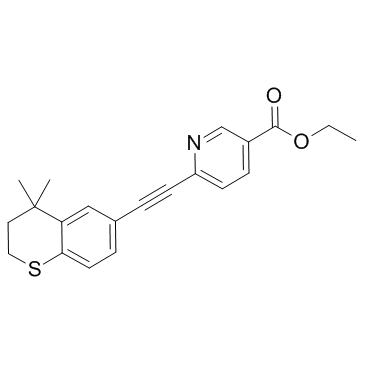 |
Tazarotene
CAS:118292-40-3 |
|
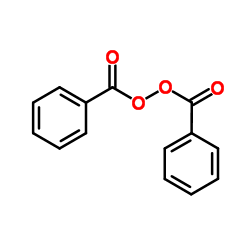 |
Benzoyl peroxide
CAS:94-36-0 |
|
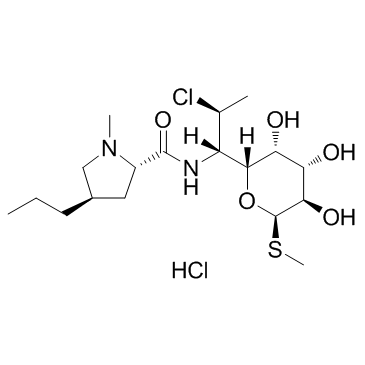 |
Clindamycin Hydrochloride
CAS:21462-39-5 |
|
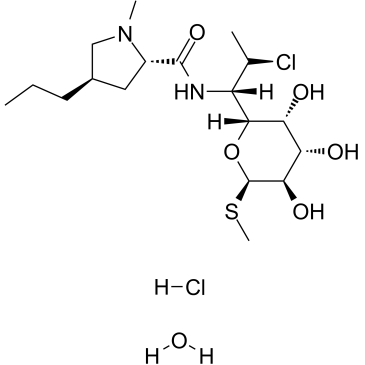 |
clindamycin hydrochloride
CAS:58207-19-5 |