| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(−)-N-Methylephedrine
CAS:552-79-4 |
|
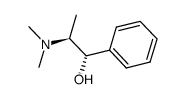 |
(1s,2s)-(+)-n-methylpseudoephedrine
CAS:51018-28-1 |
|
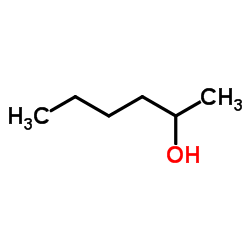 |
2-Hexanol
CAS:626-93-7 |