Synthesis of 3'-azolyl-2',3'-dideoxyhexose nucleosides.
K Walczak, E B Pedersen, C Nielsen
Index: Acta Chem. Scand. 52(7) , 935-41, (1998)
Full Text: HTML
Abstract
1,8-Diazabicyclo[5.4.0]undec-7-ene salts of 2-methyl-4(5)-nitroimidazole or benzotriazole were obtained in crystalline form. Michael-type addition of these salts to (4S,5R)-(E)-4,6-di-O-acetyl-5-hydroxy-2-hexenal gave, after acetylation of the product, an isomeric mixture of acetylated 3-(azol-1-yl)-2,3-dideoxy-D-arabino-hexopyranosides and 3-(azol-1-yl)-2,3-dideoxy-D-ribo-hexofuranosides. Reaction of these peracetylated adducts with trimethylsilylated thymine in the presence of trimethylsilyl trifluoromethanesulfonate (TMS triflate) afforded the corresponding nucleosides which were deprotected by using methanolic ammonia. The nucleosides were found inactive against HIV-1 and HSV-1.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
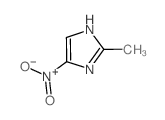 |
2-Methyl-4-nitroimidazole
CAS:696-23-1 |
C4H5N3O2 |
|
On the hydrolytic behavior of tinidazole, metronidazole, and...
2003-04-01 [J. Pharm. Sci. 92(4) , 739-46, (2003)] |
|
Radicals of nitroimidazole derivatives: pH dependence of rat...
1987-05-01 [Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 51(5) , 797-809, (1987)] |
|
[Synthesis and antibacterial activity of ciprofloxacin deriv...
2005-02-01 [Yao Xue Xue Bao 40(2) , 132-5, (2005)] |
|
[Synthesis and antibacterial activity of pyridonecarboxylic ...
2003-04-01 [Yao Xue Xue Bao 38(4) , 260-3, (2003)] |
|
[Determination of acid-base amphoteric dissociation constant...
1999-09-01 [Hua Xi Yi Ke Da Xue Xue Bao 30(3) , 345-6, (1999)] |