Synthesis of 3'-azolyl-2',3'-dideoxyhexose nucleosides.
K Walczak, E B Pedersen, C Nielsen
文献索引:Acta Chem. Scand. 52(7) , 935-41, (1998)
全文:HTML全文
摘要
1,8-Diazabicyclo[5.4.0]undec-7-ene salts of 2-methyl-4(5)-nitroimidazole or benzotriazole were obtained in crystalline form. Michael-type addition of these salts to (4S,5R)-(E)-4,6-di-O-acetyl-5-hydroxy-2-hexenal gave, after acetylation of the product, an isomeric mixture of acetylated 3-(azol-1-yl)-2,3-dideoxy-D-arabino-hexopyranosides and 3-(azol-1-yl)-2,3-dideoxy-D-ribo-hexofuranosides. Reaction of these peracetylated adducts with trimethylsilylated thymine in the presence of trimethylsilyl trifluoromethanesulfonate (TMS triflate) afforded the corresponding nucleosides which were deprotected by using methanolic ammonia. The nucleosides were found inactive against HIV-1 and HSV-1.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
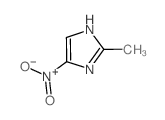 |
2-甲基-4(5)硝基咪唑
CAS:696-23-1 |
C4H5N3O2 |
|
On the hydrolytic behavior of tinidazole, metronidazole, and...
2003-04-01 [J. Pharm. Sci. 92(4) , 739-46, (2003)] |
|
Radicals of nitroimidazole derivatives: pH dependence of rat...
1987-05-01 [Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 51(5) , 797-809, (1987)] |
|
[Synthesis and antibacterial activity of ciprofloxacin deriv...
2005-02-01 [Yao Xue Xue Bao 40(2) , 132-5, (2005)] |
|
[Synthesis and antibacterial activity of pyridonecarboxylic ...
2003-04-01 [Yao Xue Xue Bao 38(4) , 260-3, (2003)] |
|
[Determination of acid-base amphoteric dissociation constant...
1999-09-01 [Hua Xi Yi Ke Da Xue Xue Bao 30(3) , 345-6, (1999)] |