| Structure | Name/CAS No. | Articles |
|---|---|---|
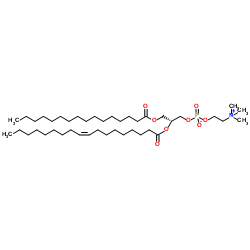 |
1-Palmitoyl-2-oleoyl-sn-glycero-3-PC
CAS:26853-31-6 |
|
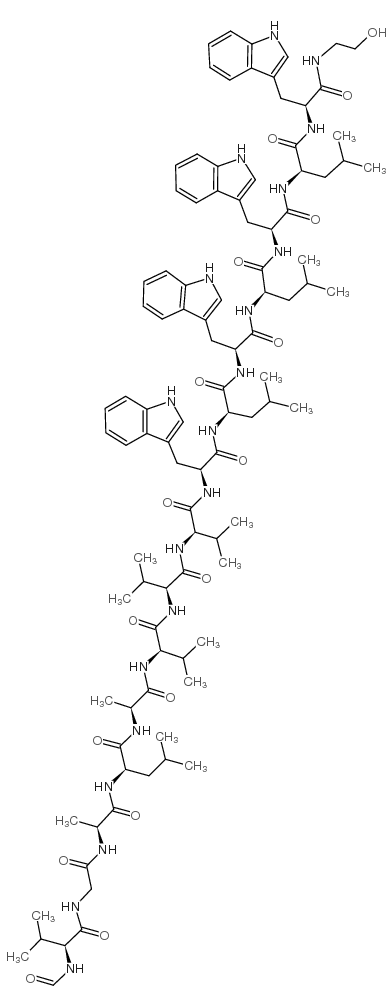 |
Gramicidin A
CAS:11029-61-1 |
|
 |
1,2-dilinoleoyl-sn-glycero-3-phosphocholine
CAS:998-06-1 |