| Structure | Name/CAS No. | Articles |
|---|---|---|
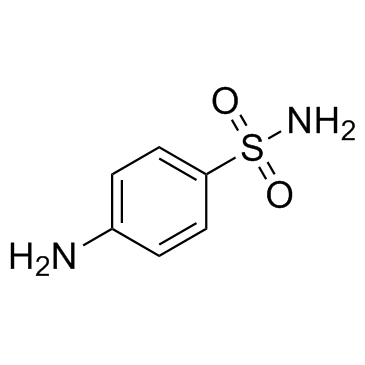 |
Sulfanilamide
CAS:63-74-1 |
|
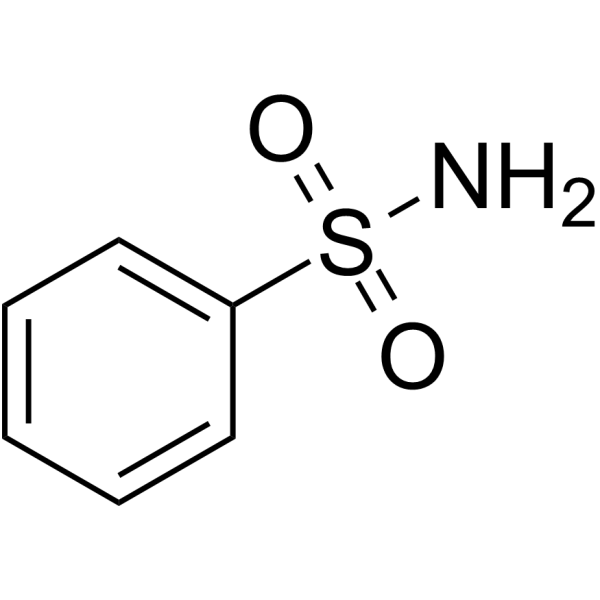 |
Benzenesulfonamide
CAS:98-10-2 |
|
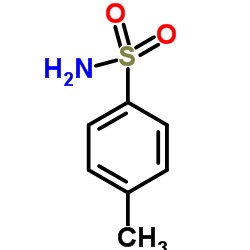 |
4-Toluenesulfonamide
CAS:70-55-3 |
|
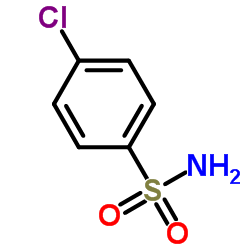 |
4-Chlorobenzenesulfonamide
CAS:98-64-6 |