| Structure | Name/CAS No. | Articles |
|---|---|---|
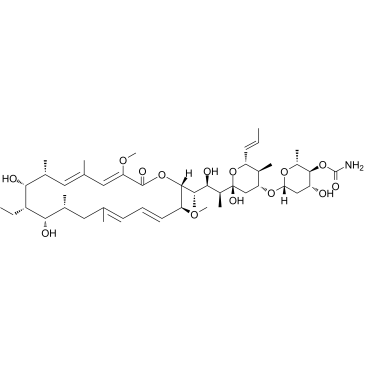 |
concanamycin A
CAS:80890-47-7 |
|
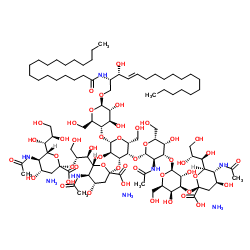 |
Ganglioside GT1b Mixture (bovine) (ammonium salt)
CAS:59247-13-1 |