concanamycin A
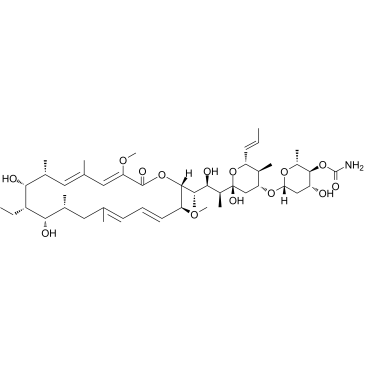
concanamycin A structure
|
Common Name | concanamycin A | ||
|---|---|---|---|---|
| CAS Number | 80890-47-7 | Molecular Weight | 866.09 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 966.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C46H75NO14 | Melting Point | 179-180℃ (dichloromethane ethanol ) | |
| MSDS | Chinese USA | Flash Point | 538.3±34.3 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of concanamycin AConcanamycin A (Antibiotic X 4357B; Concanamycin; X 4357B) is a macrolide antibiotic and a specific vacuolar type H+-ATPase (V-ATPase) inhibitor[1]. |
| Name | concanamycin A |
|---|---|
| Synonym | More Synonyms |
| Description | Concanamycin A (Antibiotic X 4357B; Concanamycin; X 4357B) is a macrolide antibiotic and a specific vacuolar type H+-ATPase (V-ATPase) inhibitor[1]. |
|---|---|
| Related Catalog | |
| Target |
Vacuolar type H+-ATPase[1] |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 966.4±65.0 °C at 760 mmHg |
| Melting Point | 179-180℃ (dichloromethane ethanol ) |
| Molecular Formula | C46H75NO14 |
| Molecular Weight | 866.09 |
| Flash Point | 538.3±34.3 °C |
| Exact Mass | 865.518738 |
| PSA | 225.92000 |
| LogP | 3.88 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.555 |
| InChIKey | DJZCTUVALDDONK-VHHURPMASA-N |
| SMILES | CC=CC1OC(O)(C(C)C(O)C(C)C2OC(=O)C(OC)=CC(C)=CC(C)C(O)C(CC)C(O)C(C)CC(C)=CC=CC2OC)CC(OC2CC(O)C(OC(N)=O)C(C)O2)C1C |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 + H310 + H330-H319 |
| Precautionary Statements | P260-P264-P280-P284-P301 + P310-P302 + P350 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+ |
| Risk Phrases | 26/27/28-36 |
| Safety Phrases | 26-36/37/39-45 |
| RIDADR | UN 3462 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | CB9732000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
2,4-Dichlorophenoxyacetic acid alters intracellular pH and ion transport in the outer mantle epithelium of the bivalve Anodonta cygnea.
Aquat. Toxicol. 154 , 12-8, (2014) Bivalve molluscs, due to their sedentary mode of life and filter-feeding behavior, are very susceptible to pollutant bioaccumulation and used as sentinel organisms in the assessment of environment pol... |
|
|
Inhibitors of vacuolar ATPase proton pumps inhibit human prostate cancer cell invasion and prostate-specific antigen expression and secretion.
Int. J. Cancer 132(2) , E1-10, (2013) Vacuolar ATPases (V-ATPases) comprise specialized and ubiquitously distributed pumps that acidify intracellular compartments and energize membranes. To gain new insights into the roles of V-ATPases in... |
|
|
Functional characterization of the Plasmodium falciparum chloroquine-resistance transporter (PfCRT) in transformed Dictyostelium discoideum vesicles.
PLoS ONE 7(6) , e39569, (2012) Chloroquine (CQ)-resistant Plasmodium falciparum malaria has been a global health catastrophe, yet much about the CQ resistance (CQR) mechanism remains unclear. Hallmarks of the CQR phenotype include ... |
| antibioticx4357b |
| S-45A |
| MFCD00210037 |
| Antibiotic A661I |
| (5R)-3-O-(4-O-Carbamoyl-2,6-dideoxy-β-D-arabino-hexopyranosyl)-2,4-dideoxy-1-C-{(2S,3R,4S)-4-[(2R,3S,4E,6E,9R,10S,11S,12R,13R,14E,16Z)-11-ethyl-10,12-dihydroxy-3,17-dimethoxy-7,9,13,15-tetramethyl-18-oxooxacyclooctadeca-4,6,14,16-tetraen-2-yl]-3-hydroxypentan-2-yl}-4-methyl-5-[(1E)-prop-1-en-1-yl]-α-D-threo-pentopyranose |
| FOLIMYCIN,STREPTOMYCES SP |
| α-D-threo-Pentopyranose, 3-O-[4-O-(aminocarbonyl)-2,6-dideoxy-β-D-arabino-hexopyranosyl]-2,4-dideoxy-1-C-[(1S,2R,3S)-3-[(2R,3S,4E,6E,9R,10S,11S,12R,13R,14E,16Z)-11-ethyl-10,12-dihydroxy-3,17-dimethoxy-7,9,13,15-tetramethyl-18-oxooxacyclooctadeca-4,6,14,16-tetraen-2-yl]-2-hydroxy-1-methylbutyl]-4-methyl-5-C-[(1E)-1-propen-1-yl]-, (5R)- |
| A661-I |
| FOLIMYCIN |
| Antibiotic S-45A |
| x4357b |
| α-D-threo-Pentopyranose, 3-O-[4-O-(aminocarbonyl)-2,6-dideoxy-β-D-arabino-hexopyranosyl]-2,4-dideoxy-1-C-[(1S,2R,3S)-3-[(2R,3S,4E,6E,9R,10S,11S,12R,13R,14E,16Z)-11-ethyl-10,12-dihydroxy-3,17-di methoxy-7,9,13,15-tetramethyl-18-oxooxacyclooctadeca-4,6,14,16-tetraen-2-yl]-2-hydroxy-1-methylbutyl]-4-methyl-5-C-[(1E)-1-propen-1-yl]-, (5R)- |
| Concanamycin A,(3Z,5E,7R,8R,9S,10S,11R,13E,15E,17S,18R)-18-[(1S,2R,3S)-3-[(2R,4R,5S,6R)-4-[[4-O-(Aminocarbonyl)-2,6-dideoxy-β-D-arabino-hexopyranosyl]oxy]tetrahydro-2-hydroxy-5-methyl-6-(1E)-1-propenyl-2H-pyran-2-yl]-2-hydroxy-1-methylbutyl]-9-ethyl-8,10- |
| CONCANAMYCIN A |
| (5R)-3-O-(4-O-Carbamoyl-2,6-dideoxy-β-D-arabino-hexopyranosyl)-2,4-dideoxy-1-C-{(2S,3R,4S)-4-[(2R,3S,4E,6E,9R,10S,11S,12R,13R,14E,16Z)-11-ethyl-10,12-dihydroxy-3,17-dimethoxy-7,9,13,15-tetramethyl- 18-oxooxacyclooctadeca-4,6,14,16-tetraen-2-yl]-3-hydroxy-2-pentanyl}-4-methyl-5-[(1E)-1-propen-1-yl]-α-D-threo-pentopyranose |

