| Structure | Name/CAS No. | Articles |
|---|---|---|
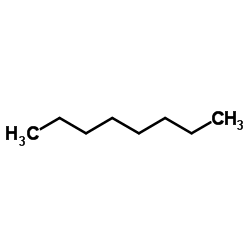 |
Octane
CAS:111-65-9 |
|
 |
Steviol
CAS:471-80-7 |
|
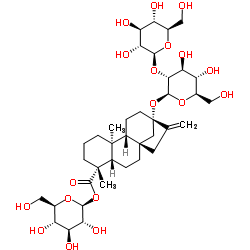 |
Stevioside
CAS:57817-89-7 |