Binding of heparin to antithrombin III: a chemical proof of the critical role played by a 3-sulfated 2-amino-2-deoxy-D-glucose residue.
M Petitou, P Duchaussoy, I Lederman, J Choay, P Sinaÿ
Index: Carbohydr. Res. 179 , 163-72, (1988)
Full Text: HTML
Abstract
Known methyl (prop-1-enyl 2,3-di-O-benzyl-alpha-D-glucopyranosid)uronate was first converted into methyl (prop-1-enyl 2,3-di-O-benzyl-4-O-levulinyl-alpha-D-gluco-pyranosid)uro nat e. Acid hydrolysis, followed by treatment with (bromomethylene)-dimethylammonium bromide, gave methyl (2,3-di-O-benzyl-4-O-levulinyl-alpha-D-glucopyranosyl bromide)uronate. Condensation of this bromide with 1,6-anhydro-2-azido-3-O-benzyl-2-deoxy-beta-D-glucopyranose gave 1,6-anhydro-2-azido-3-O-benzyl-2-deoxy-4-O-(methyl 2,3-di-O-benzyl-4-O- levulinyl-beta-D-glucopyranosyluronate)-beta-D-glucopyranose. Acetolysis, followed by selective anomeric O-deacetylation and treatment with (bromomethylene)dimethylammonium bromide then gave 6-O-acetyl-2-azido-3-O-benzyl-2-deoxy-4-O-(methyl 2,3-di-O-benzyl-4-O-levulinyl -beta-D-glucopyranosyluronate)-alpha-D-glucopyranosyl bromide. Condensation of this bromide with benzyl 6-O-acetyl-3-O-benzyl-2-benzyloxycarbonylamino-2-deoxy-4- O-(methyl 2-O-acetyl-3-O-benzyl-alpha-L-idopyranosyluronate)-alpha-D- glucopyranoside provided benzyl O-(methyl 2,3-di-O-benzyl-4-O-levulinyl-beta-D- glucopyranosyluronate)-(1----4)-O-(6-O-acetyl-2-azido-3-O-benzyl-2-deoxy - alpha-D-glucopyranosyl)- (1----4)-O-(methyl 2-O-acetyl-3-O-benzyl-alpha-L-idopyranosyluronate)-(1----4)- 6-O-acetyl-3-O-benzyl-2-benzyloxycarbonylamino-2-deoxy-alpha-D-glu copyranoside. Removal of the levulinyl group followed by condensation with 6-O-acetyl-2-azido-3,4-di-O -benzyl-2-deoxy-alpha-D-glucopyranosyl bromide provided benzyl O-(6-O-acetyl-2- azido-3,4-di-O-benzyl-2-deoxy-alpha-D-glucopyranosyl)-(1----4)-O-(methyl 2,3-di- O-benzyl-beta-D-glucopyranosyluronate)-(1----4)-O-(6-O-acetyl-2-azido-3- O- benzyl-2- deoxy-alpha-D-glucopyranosyl)-(1----4)-O-(methyl 2-O-acetyl-3-O-benzyl-alpha-L- idopyranosyluronate)-(1----4)-6-O-acetyl-3-O-benzyl-2-benzyloxycarbon ylamino-2- deoxy-alpha-D-glucopyranoside in 78% yield. O-Deacetylation followed by re-esterification, O-sulfation, catalytic hydrogenolysis, saponification, and N-sulfation gave the non-sodium salt of O-(2-deoxy-6-O-sulfo-2-sulfoamino-alpha-D-glucopyranosyl)-(1----4) -O- (beta-D-glucopyranosyluronic acid)-(1----4)O-(2-deoxy-6-O-sulfo-2-sulfoamino- alpha-D-glucopyranosyl)-(1----4)-O-(2-O-sulfo-alpha-L-idopyranosyluronic acid)- (1----4)-2-deoxy-6-O-sulfo-2-sulfoamino-D-glucopyranose. This synthetic pentasaccharide neither binds to antithrombin III nor induces anti-factor Xa activity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
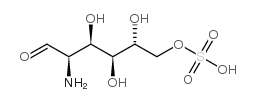 |
D-Glucosamine-6-sulfate
CAS:91674-26-9 |
C6H13NO8S |
|
Human ficolin-2 recognition versatility extended: an update ...
2014-12-20 [FEBS Lett. 588(24) , 4694-700, (2014)] |
|
Characteristics of the glmS ribozyme suggest only structural...
2006-04-01 [RNA 12 , 607 - 619, (2006)] |
|
The effects of glucosamine sulfate on intervertebral disc an...
2015-06-01 [Spine J. 15 , 1339-46, (2015)] |
|
Common binding sites for beta-amyloid fibrils and fibroblast...
1999-10-22 [J. Biol. Chem. 274(43) , 30631-5, (1999)] |
|
Anti-obesity effect of sulfated glucosamine by AMPK signal p...
2009-10-01 [Food Chem. Toxicol. 47(10) , 2401-6, (2009)] |