| Structure | Name/CAS No. | Articles |
|---|---|---|
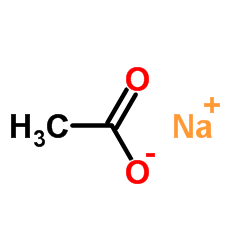 |
Sodium acetate
CAS:127-09-3 |
|
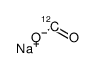 |
sodium,oxomethanolate
CAS:1218765-26-4 |
|
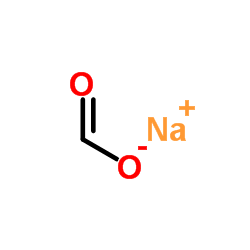 |
Sodium formate
CAS:141-53-7 |
|
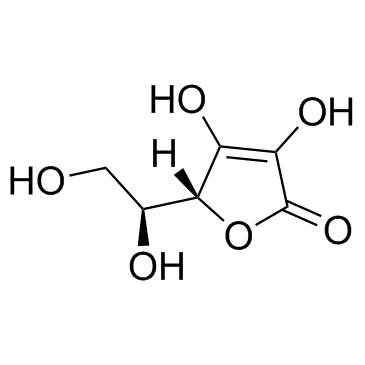 |
Ascorbic acid
CAS:50-81-7 |
|
 |
Water
CAS:7732-18-5 |
|
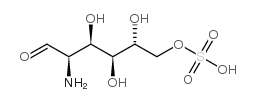 |
D-Glucosamine-6-sulfate
CAS:91674-26-9 |
|
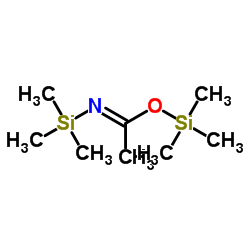 |
N,O-Bis(trimethylsilyl)acetamide
CAS:10416-59-8 |
|
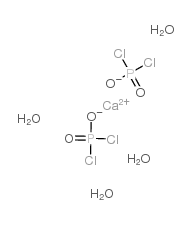 |
Phosphocholine Chloride Calcium Salt Tetrahydrate
CAS:72556-74-2 |