| Structure | Name/CAS No. | Articles |
|---|---|---|
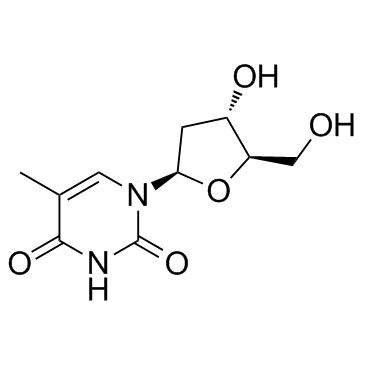 |
Thymidine
CAS:50-89-5 |
|
 |
SANT-1
CAS:304909-07-7 |
|
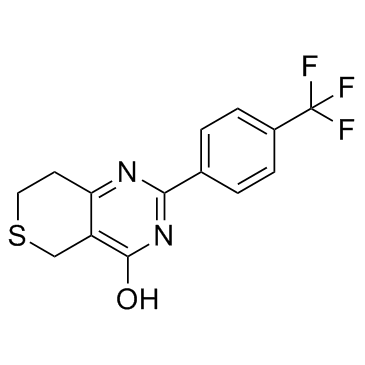 |
XAV-939
CAS:284028-89-3 |
|
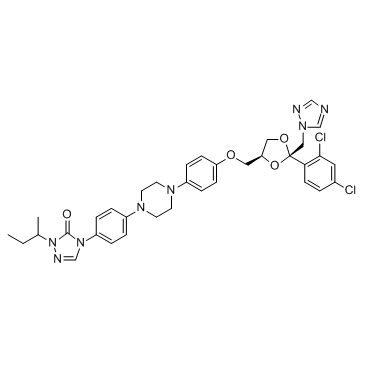 |
Itraconazole
CAS:84625-61-6 |