| Structure | Name/CAS No. | Articles |
|---|---|---|
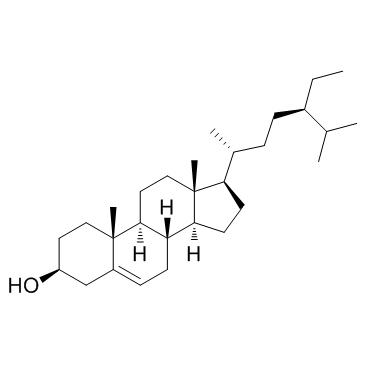 |
Beta-Sitosterol
CAS:83-46-5 |
|
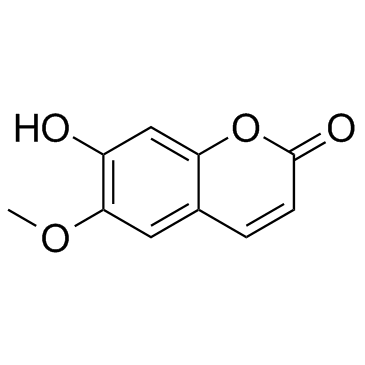 |
Scopoletin
CAS:92-61-5 |
|
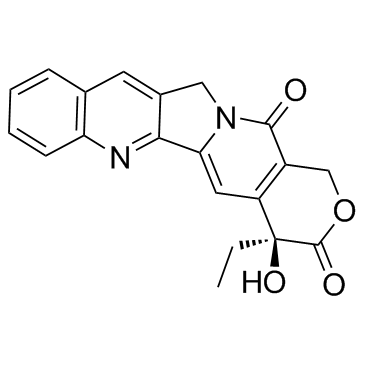 |
Campathecin
CAS:7689-03-4 |
|
 |
Cinnamic acid
CAS:140-10-3 |
|
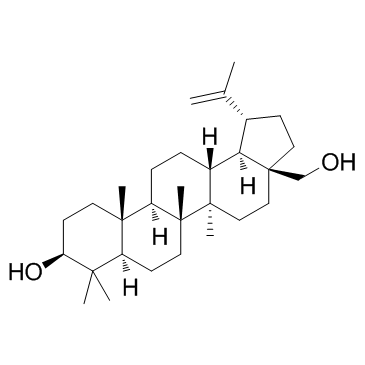 |
Betulin
CAS:473-98-3 |
|
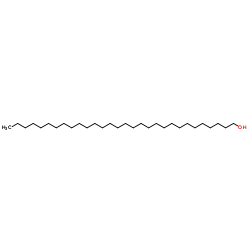 |
1-Triacontanol
CAS:593-50-0 |
|
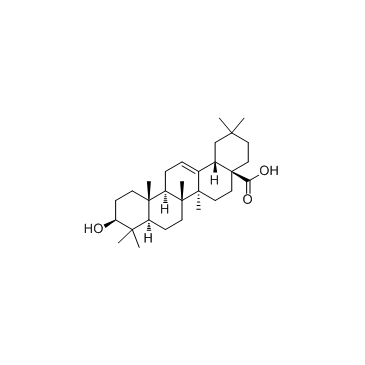 |
Oleanic acid
CAS:508-02-1 |
|
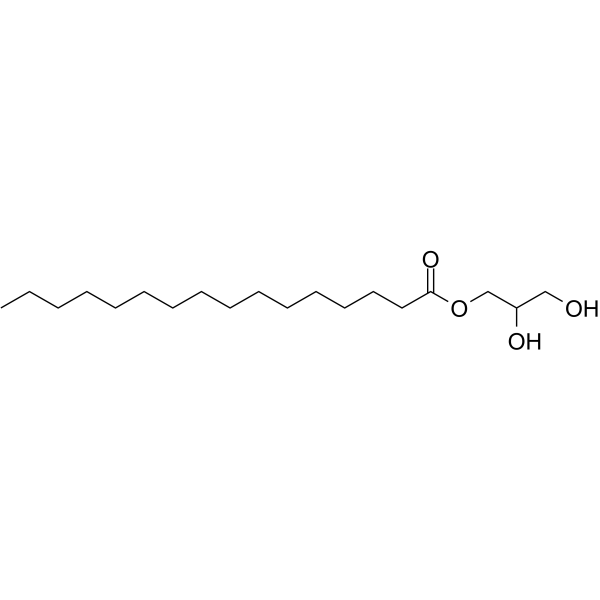 |
1-Monopalmitin
CAS:542-44-9 |