| Structure | Name/CAS No. | Articles |
|---|---|---|
2 Structure](https://image.chemsrc.com/caspic/066/134668-73-8.png) |
[Pd(dppp)(H2O)2](BF4)2
CAS:134668-73-8 |
|
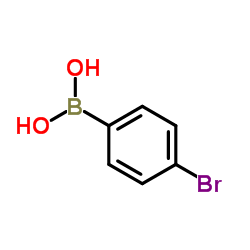 |
4-Bromophenylboronic Acid
CAS:5467-74-3 |
|
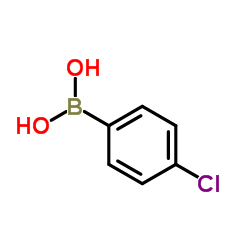 |
(4-Chlorophenyl)boronic acid
CAS:1679-18-1 |