| Structure | Name/CAS No. | Articles |
|---|---|---|
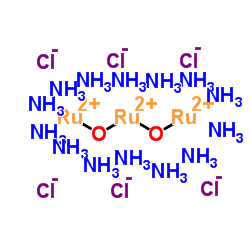 |
Ruthenium red
CAS:11103-72-3 |
|
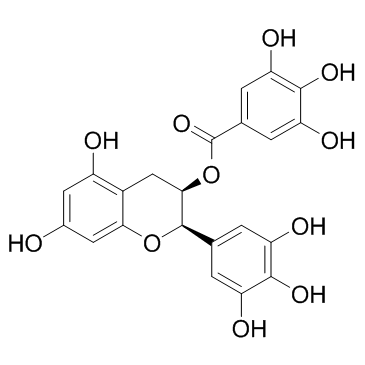 |
(-)-Epigallocatechin gallate
CAS:989-51-5 |
|
 |
POTASSIUM PECTATE
CAS:37251-70-0 |