| Structure | Name/CAS No. | Articles |
|---|---|---|
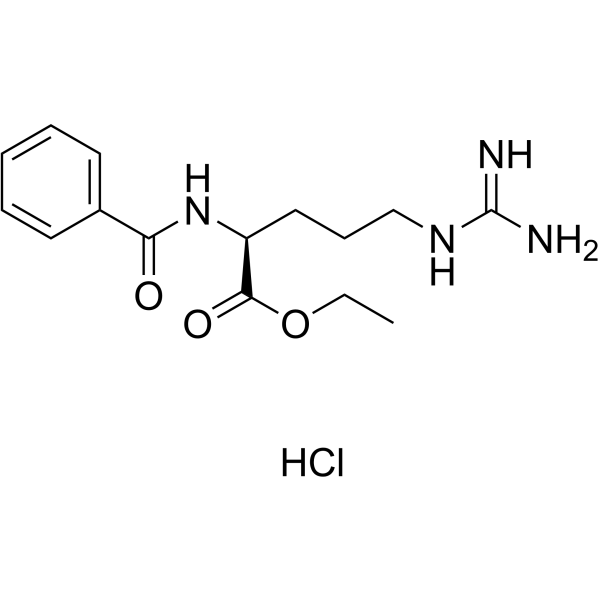 |
Bz-Arg-OEt��HCl
CAS:2645-08-1 |
|
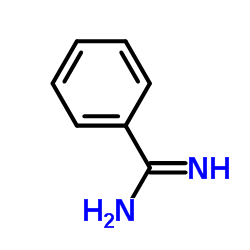 |
Benzamidine
CAS:618-39-3 |
|
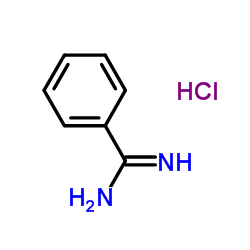 |
Benzimidamide hydrochloride
CAS:1670-14-0 |
|
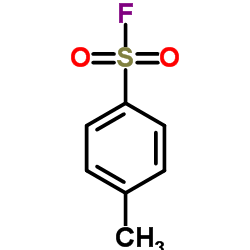 |
p-toluenesulfonyl fluoride
CAS:455-16-3 |