| Structure | Name/CAS No. | Articles |
|---|---|---|
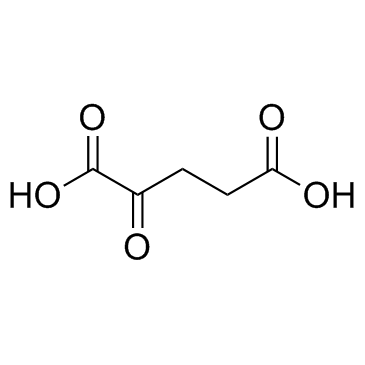 |
2-Ketoglutaric acid
CAS:328-50-7 |
|
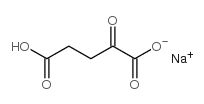 |
Alpha-Ketoglutaric acid sodium salt
CAS:22202-68-2 |
|
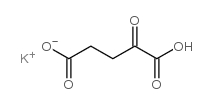 |
Potassium hydrogen 2-oxoglutarate
CAS:997-43-3 |
|
 |
α-Ketoglutarate dehydrogenase
CAS:9031-02-1 |