| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
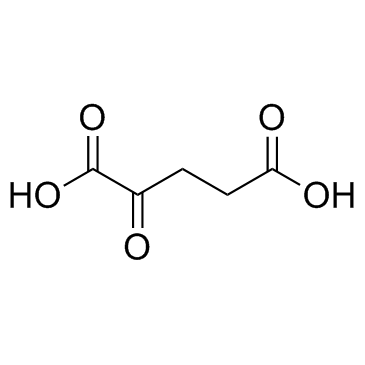 |
α-酮戊二酸
CAS:328-50-7 |
|
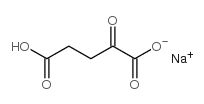 |
LPHA-酮戊二酸钠
CAS:22202-68-2 |
|
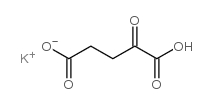 |
α-酮戊二酸单钾盐
CAS:997-43-3 |
|
 |
α-酮戊二酸脱氢酶 来源于猪心脏
CAS:9031-02-1 |