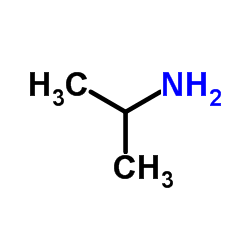
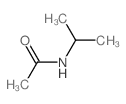
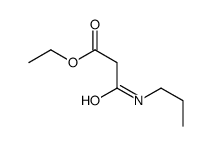
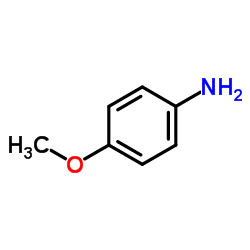
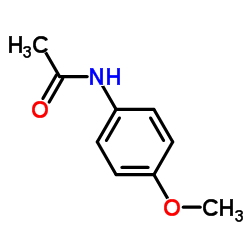
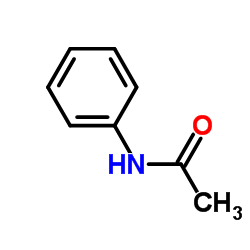
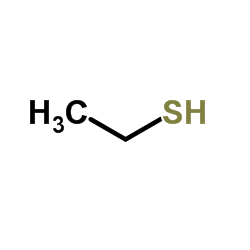
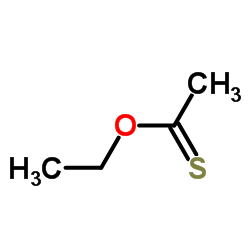
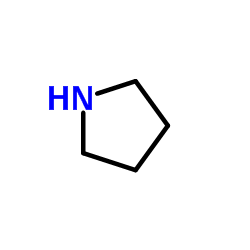
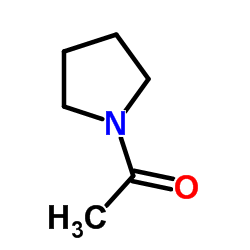
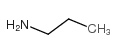
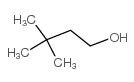
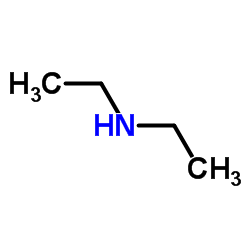
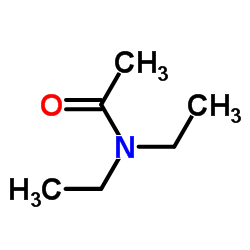
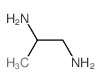
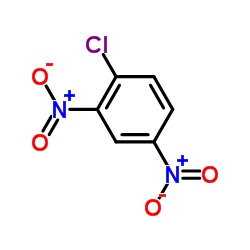
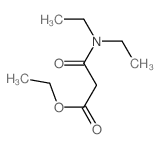
|
~89% |
|
~% |
|
~95% |
|
~% |
|
~% |
|
~98% |
|
~76% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~92% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~% |
|
~64% |
|
~98% |
|
~75% |
|
~% |
|
~99% |
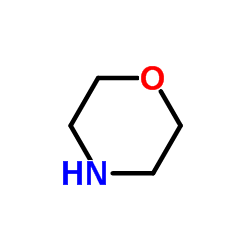

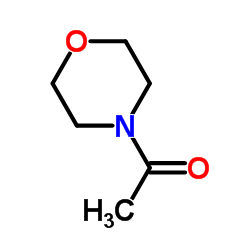
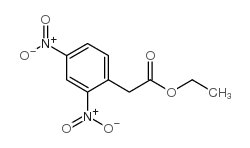
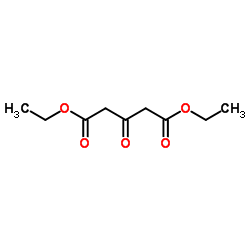
![Propanoic acid,3-[(1,1-dimethylethyl)amino]-3-oxo-, ethyl ester Structure](https://image.chemsrc.com/caspic/216/17797-87-4.png)