Characterization and functional modification of StaC and RebC, which are involved in the pyrrole oxidation of indolocarbazole biosynthesis.
Shumpei Asamizu, Yoshitsugu Shiro, Yasuhiro Igarashi, Shingo Nagano, Hiroyasu Onaka
Index: Biosci. Biotechnol. Biochem. 75(11) , 2184-93, (2011)
Full Text: HTML
Abstract
The diversity of indolocarbazole natural products results from the differences in oxidation states of the pyrroline ring moiety. In the biosynthetic pathways for staurosporine and rebeccamycin, two homologous enzymes having 64% identity, StaC and RebC, are responsible for the selective production of K252c, which has one oxo group at the pyrroline ring, and arcyriaflavin A, which has two. Although StaC has a FAD-binding motif, most StaC molecules do not contain FAD, and the protein cannot be reconstituted with FAD in vitro. In this study, we mutated Ala-118 in StaC by replacing a glutamine that is conserved in FAD monooxygenases, resulting in increased FAD content as well as catalytic activity. In addition, mutations around the substrate-binding sites of StaC and RebC can change the product selectivity. Specifically, StaC-N244R-V246T and RebC-F216V-R239N mutants produced substantial amounts of arcyriaflavin A and K252c, respectively.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
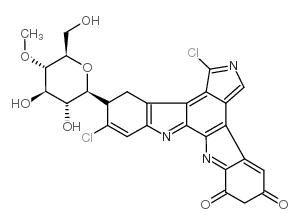 |
Rebeccamycin
CAS:93908-02-2 |
C27H21Cl2N3O7 |
|
Structure and mechanism of the rebeccamycin sugar 4'-O-methy...
2008-08-15 [J. Biol. Chem. 283(33) , 22628-36, (2008)] |
|
Natural product diversification using a non-natural cofactor...
2006-03-08 [J. Am. Chem. Soc. 128(9) , 2760-1, (2006)] |
|
Engineering biosynthetic pathways to generate antitumor indo...
2006-07-01 [J. Ind. Microbiol. Biotechnol. 33(7) , 560-8, (2006)] |
|
Flavin redox chemistry precedes substrate chlorination durin...
2006-06-27 [Biochemistry 45(25) , 7904-12, (2006)] |
|
Rebeccamycin derivatives as dual DNA-damaging agents and pot...
2008-12-01 [Mol. Pharmacol. 74(6) , 1620-9, (2008)] |