Natural product diversification using a non-natural cofactor analogue of S-adenosyl-L-methionine.
Changsheng Zhang, Rachel L Weller, Jon S Thorson, Scott R Rajski
Index: J. Am. Chem. Soc. 128(9) , 2760-1, (2006)
Full Text: HTML
Abstract
Adenosine analogues bearing either 5'-aziridine or 5'-N-mustard electrophiles are methyltransferase-dependent DNA alkylating agents. We present here a novel synthetic cofactor bearing a pendant 5'-amino acid N-mustard. Unlike previously studied synthetic cofactors, this material is very efficiently used by the natural product biosynthetic enzyme rebeccamycin methyltransferase (RebM) to generate a number of new rebeccamycin analogues. These data promote the notion that natural product methyltransferases can be used with non-natural cofactors to enhance the molecular diversity of natural product analogues for drug discovery. To our knowledge, this is the first documentation of a biological methyltransferase, other than DNA methyltransferases, that can exploit such synthetic cofactors.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
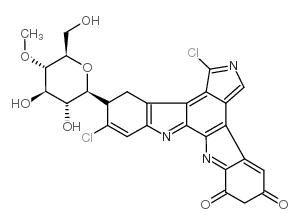 |
Rebeccamycin
CAS:93908-02-2 |
C27H21Cl2N3O7 |
|
Structure and mechanism of the rebeccamycin sugar 4'-O-methy...
2008-08-15 [J. Biol. Chem. 283(33) , 22628-36, (2008)] |
|
Engineering biosynthetic pathways to generate antitumor indo...
2006-07-01 [J. Ind. Microbiol. Biotechnol. 33(7) , 560-8, (2006)] |
|
Flavin redox chemistry precedes substrate chlorination durin...
2006-06-27 [Biochemistry 45(25) , 7904-12, (2006)] |
|
Rebeccamycin derivatives as dual DNA-damaging agents and pot...
2008-12-01 [Mol. Pharmacol. 74(6) , 1620-9, (2008)] |
|
Molecular cloning, sequence analysis and functional characte...
2009-10-01 [Mol. Biosyst. 5(10) , 1180-91, (2009)] |