Engineering biosynthetic pathways to generate antitumor indolocarbazole derivatives.
César Sánchez, Carmen Méndez, José A Salas
Index: J. Ind. Microbiol. Biotechnol. 33(7) , 560-8, (2006)
Full Text: HTML
Abstract
The indolocarbazole family of natural products is a source of lead compounds with potential therapeutic applications in the treatment of cancer and neurodegenerative disorders. Rebeccamycin and staurosporine are two members of this family, which are produced by different actinomycete strains. Although both compounds display antitumor activity, their distinct structural features determine different modes of action: rebeccamycin targets DNA topoisomerase I, while staurosporine is a protein kinase inhibitor. Here we examine the biosyntheses of rebeccamycin and staurosporine while we summarize our recent work concerning (a) identification and characterization of genes involved in the biosynthesis of indolocarbazoles in actinomycetes, and (b) generation of novel indolocarbazole derivatives in microorganisms by combinatorial biosynthesis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
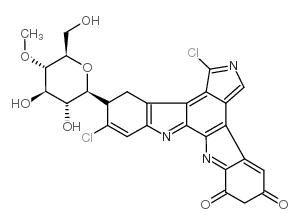 |
Rebeccamycin
CAS:93908-02-2 |
C27H21Cl2N3O7 |
|
Structure and mechanism of the rebeccamycin sugar 4'-O-methy...
2008-08-15 [J. Biol. Chem. 283(33) , 22628-36, (2008)] |
|
Natural product diversification using a non-natural cofactor...
2006-03-08 [J. Am. Chem. Soc. 128(9) , 2760-1, (2006)] |
|
Flavin redox chemistry precedes substrate chlorination durin...
2006-06-27 [Biochemistry 45(25) , 7904-12, (2006)] |
|
Rebeccamycin derivatives as dual DNA-damaging agents and pot...
2008-12-01 [Mol. Pharmacol. 74(6) , 1620-9, (2008)] |
|
Molecular cloning, sequence analysis and functional characte...
2009-10-01 [Mol. Biosyst. 5(10) , 1180-91, (2009)] |