Rebeccamycin derivatives as dual DNA-damaging agents and potent checkpoint kinase 1 inhibitors.
Christelle Marminon, Fabrice Anizon, Pascale Moreau, Bruno Pfeiffer, Alain Pierré, Roy M Golsteyn, Paul Peixoto, Marie-Paule Hildebrand, Marie-Hélène David-Cordonnier, Olivier Lozach, Laurent Meijer, Michelle Prudhomme
Index: Mol. Pharmacol. 74(6) , 1620-9, (2008)
Full Text: HTML
Abstract
Rebeccamycin is an indolocarbazole class inhibitor of topoisomerase I. In the course of structure-activity relationship studies on rebeccamycin derivatives, we have synthesized analogs with the sugar moiety attached to either one or both indole nitrogens. Some analogs, especially those with substitutions at the 6' position of the carbohydrate moiety, exhibit potent inhibitory activity toward checkpoint kinase 1 (Chk1), a kinase that has a major role in the G(2)/M checkpoint in response to DNA damage. Some of these compounds retained a genotoxic activity either through intercalation into the DNA and/or by topoisomerase I-mediated DNA cleavage. We explored the structure-activity relationship between these compounds and their multiple targets. These rebeccamycin derivatives represent a novel class of potential antitumor agents that have a dual effect and might selectively induce the death of cancer cells.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
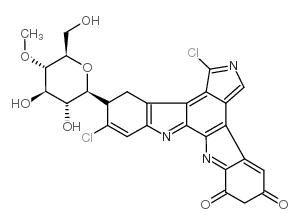 |
Rebeccamycin
CAS:93908-02-2 |
C27H21Cl2N3O7 |
|
Structure and mechanism of the rebeccamycin sugar 4'-O-methy...
2008-08-15 [J. Biol. Chem. 283(33) , 22628-36, (2008)] |
|
Natural product diversification using a non-natural cofactor...
2006-03-08 [J. Am. Chem. Soc. 128(9) , 2760-1, (2006)] |
|
Engineering biosynthetic pathways to generate antitumor indo...
2006-07-01 [J. Ind. Microbiol. Biotechnol. 33(7) , 560-8, (2006)] |
|
Flavin redox chemistry precedes substrate chlorination durin...
2006-06-27 [Biochemistry 45(25) , 7904-12, (2006)] |
|
Molecular cloning, sequence analysis and functional characte...
2009-10-01 [Mol. Biosyst. 5(10) , 1180-91, (2009)] |