| Structure | Name/CAS No. | Articles |
|---|---|---|
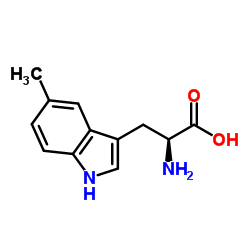 |
5-Methyltryptophan
CAS:951-55-3 |
|
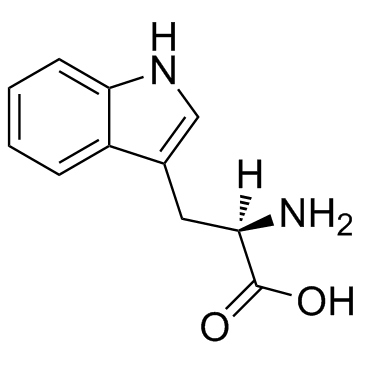 |
D-tryptophan
CAS:153-94-6 |
|
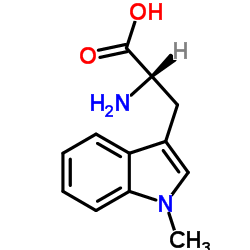 |
(S)-Indoximod
CAS:21339-55-9 |
|
 |
5-Methoxytryptophan
CAS:28052-84-8 |