| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
L(-)-Tryptophan
CAS:73-22-3 |
|
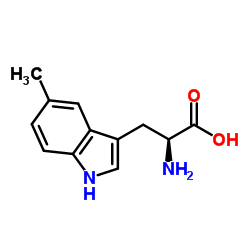 |
5-Methyltryptophan
CAS:951-55-3 |
|
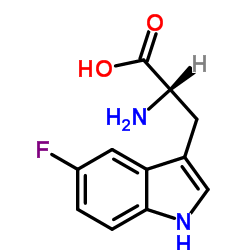 |
DL-5-Fluorotryptophan
CAS:16626-02-1 |
|
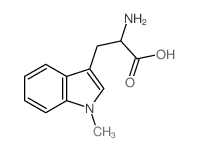 |
(Rac)-Indoximod
CAS:26988-72-7 |
|
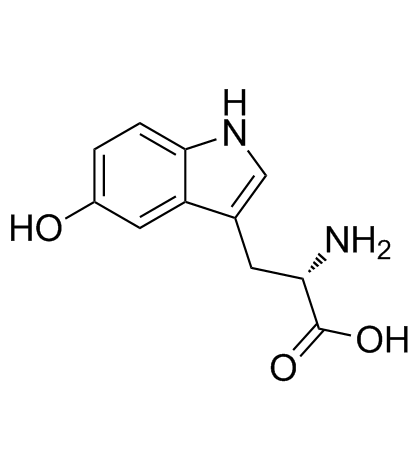 |
L-5-Hydroxytryptophan
CAS:4350-09-8 |
|
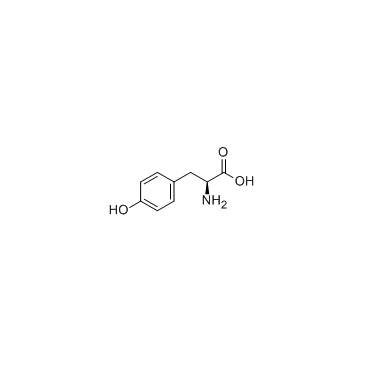 |
L-Tyrosine
CAS:60-18-4 |
|
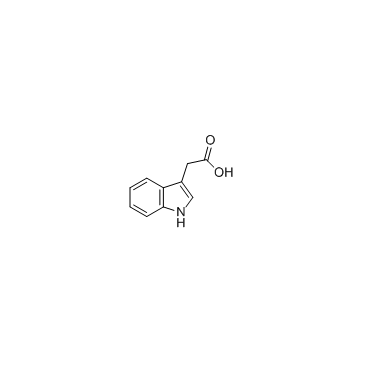 |
3-Indoleacetic acid
CAS:87-51-4 |
|
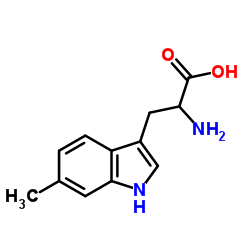 |
6-Methyltryptophan
CAS:2280-85-5 |
|
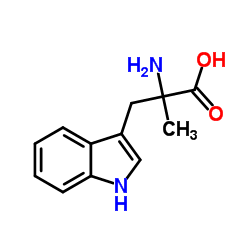 |
α-Methyltryptophan
CAS:153-91-3 |
|
 |
L-(+)-Abrine
CAS:526-31-8 |