(S)-Indoximod
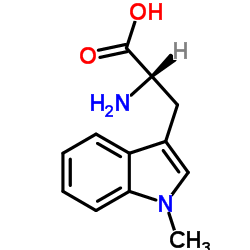
(S)-Indoximod structure
|
Common Name | (S)-Indoximod | ||
|---|---|---|---|---|
| CAS Number | 21339-55-9 | Molecular Weight | 218.252 | |
| Density | 1.28 | Boiling Point | 429.3±35.0 °C at 760 mmHg | |
| Molecular Formula | C12H14N2O2 | Melting Point | 223-226 ºC | |
| MSDS | Chinese USA | Flash Point | 213.4±25.9 °C | |
Use of (S)-Indoximod(S)-Indoximod (1-Methyl-L-tryptophan) is an inhibitor of indoleamine 2,3-dioxygenase (IDO). (S)-Indoximod can be used for the research of cancer[1][2]. |
| Name | (2S)-2-amino-3-(1-methylindol-3-yl)propanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-Indoximod (1-Methyl-L-tryptophan) is an inhibitor of indoleamine 2,3-dioxygenase (IDO). (S)-Indoximod can be used for the research of cancer[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Indoleamine 2,3-Dioxygenase (IDO)[1] |
| References |
| Density | 1.28 |
|---|---|
| Boiling Point | 429.3±35.0 °C at 760 mmHg |
| Melting Point | 223-226 ºC |
| Molecular Formula | C12H14N2O2 |
| Molecular Weight | 218.252 |
| Flash Point | 213.4±25.9 °C |
| Exact Mass | 218.105530 |
| PSA | 68.25000 |
| LogP | 1.43 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.625 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~92% 
(S)-Indoximod CAS#:21339-55-9 |
| Literature: He, Bin; Song, Hao; Du, Yu; Qin, Yong Journal of Organic Chemistry, 2009 , vol. 74, # 1 p. 298 - 304 |
|
~% 
(S)-Indoximod CAS#:21339-55-9 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 12, # 17 p. 2471 - 2474 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Human renal tubular epithelial cells suppress alloreactive T cell proliferation.
Clin. Exp. Immunol. 179(3) , 509-19, (2015) Renal tubular epithelial cells (TECs) are one of the main targets of alloreactive T cells during acute rejection. We hypothesize that TECs modulate the outcome of alloimmunity by executing immunosuppr... |
|
|
Induction of indoleamine 2,3-dioxygenase (IDO) enzymatic activity contributes to interferon-gamma induced apoptosis and death receptor 5 expression in human non-small cell lung cancer cells.
Asian Pac. J. Cancer Prev. 15(18) , 7995-8001, (2014) Interferon-gamma (IFN-γ) has been used to treat various malignant tumors. However, the molecular mechanisms underlying the direct anti-proliferative activity of IFN-γ are poorly understood. In the pre... |
|
|
Interferon gamma-mediated BoHV-4 replication restriction in bovine endometrial stromal cells is host IDO1 gene expression independent and BoHV-4 IE2 gene expression dependent.
Biol. Reprod. 91(5) , 112, (2014) In the present work the interaction between bovine herpesvirus 4 (BoHV-4)-infected bovine endometrial stromal cells (BESCs) and interferon gamma (IFNG) was investigated. Starting from the particular t... |
| 1-Methyl-L-tryptophan |
| DL-1-Methyltryptophan |
| MFCD00467133 |
| L-Tryptophan, 1-methyl- |
| 1-methyl-(D,L)-tryptophan |
| 1-Methyl-L-tryptophane |
| H-L-Trp(N-CH3)-OH |
| H-Trp(1-Me)-OH |
| BIDD:GT0440 |
| (S)-2-Amino-3-(1-methyl-1H-indol-3-yl)propanoic acid |



