| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Boc-L-phenylalanine
CAS:13734-34-4 |
|
![N-[(tert-Butoxy)carbonyl]-L-tryptophan Structure](https://image.chemsrc.com/caspic/447/13139-14-5.png) |
N-[(tert-Butoxy)carbonyl]-L-tryptophan
CAS:13139-14-5 |
|
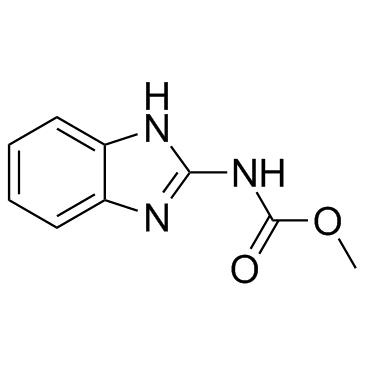 |
Carbendazim
CAS:10605-21-7 |
|
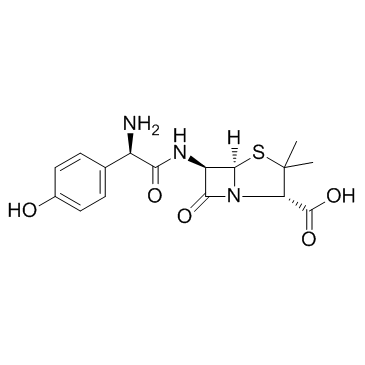 |
Amoxicillin
CAS:26787-78-0 |