| Structure | Name/CAS No. | Articles |
|---|---|---|
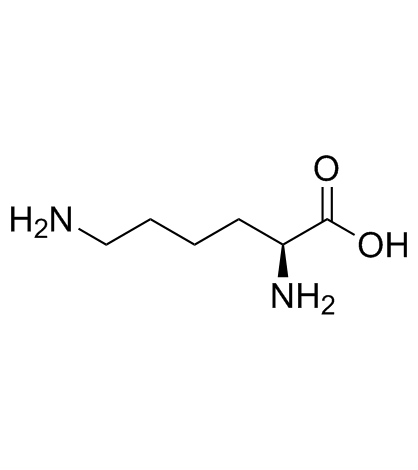 |
L-Lysine
CAS:56-87-1 |
|
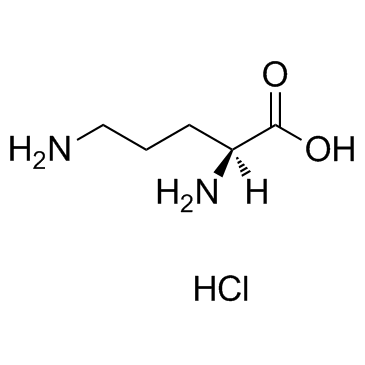 |
L(+)-Ornithine hydrochloride
CAS:3184-13-2 |
|
 |
L-(+)-Lysine monohydrochloride
CAS:657-27-2 |
|
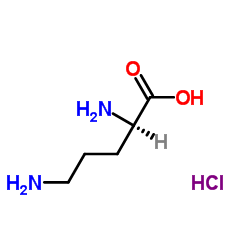 |
D-Ornithine monohydrochloride
CAS:16682-12-5 |
|
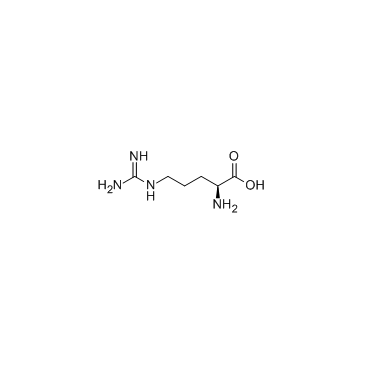 |
L-arginine
CAS:74-79-3 |
|
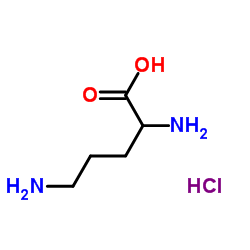 |
H-DL-Orn-OH.HCl
CAS:1069-31-4 |
|
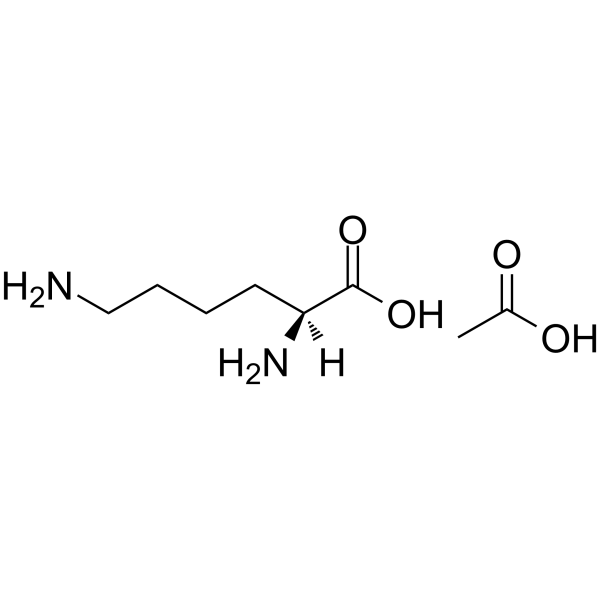 |
L-Lysine monoacetate
CAS:57282-49-2 |
|
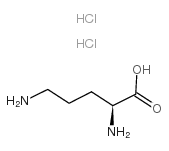 |
L-Ornithine,hydrochloride (1:2)
CAS:6211-16-1 |
|
 |
GHRP-6 Acetate
CAS:87616-84-0 |