| Structure | Name/CAS No. | Articles |
|---|---|---|
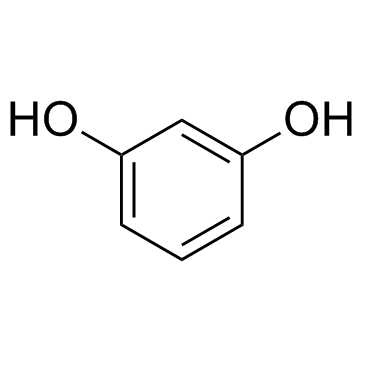 |
Resorcine
CAS:108-46-3 |
|
 |
Salicylic acid
CAS:69-72-7 |
|
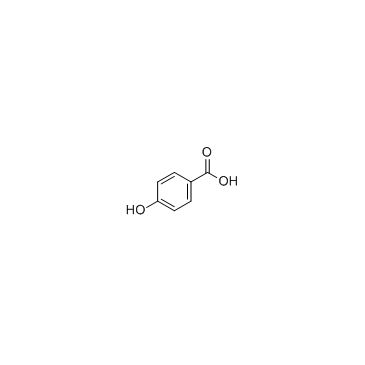 |
4-Hydroxybenzoic acid
CAS:99-96-7 |
|
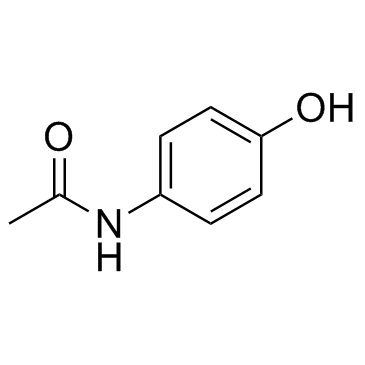 |
4-Acetamidophenol
CAS:103-90-2 |
|
 |
Phenol
CAS:108-95-2 |
|
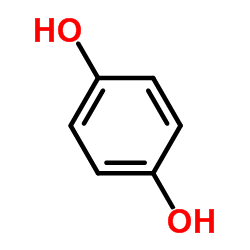 |
Hydroquinone
CAS:123-31-9 |
|
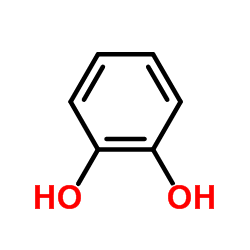 |
1,2-Benzenediol
CAS:120-80-9 |
|
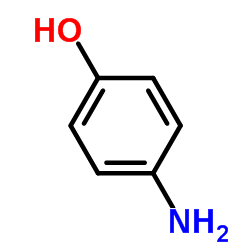 |
4-Aminophenol
CAS:123-30-8 |
|
 |
Clioquinol
CAS:130-26-7 |
|
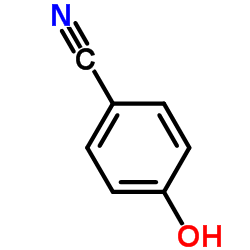 |
4-Hydroxybenzonitrile
CAS:767-00-0 |