| Structure | Name/CAS No. | Articles |
|---|---|---|
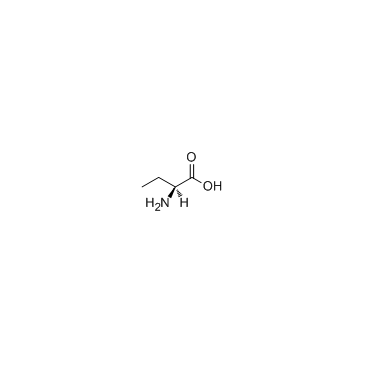 |
L(+)-2-Aminobutyric acid
CAS:1492-24-6 |
|
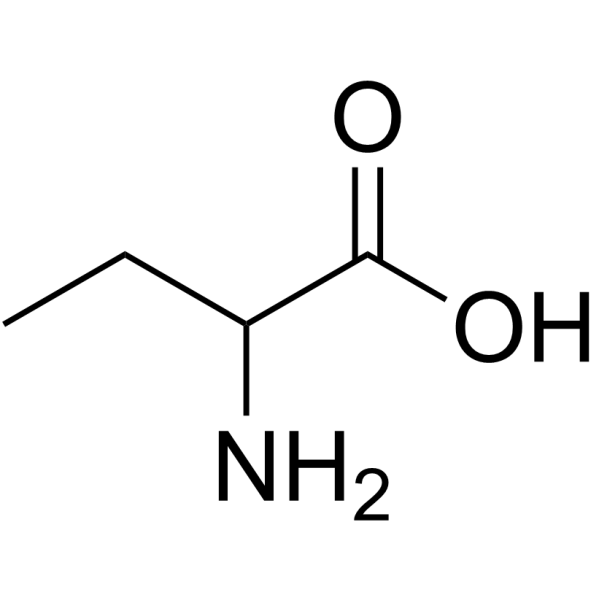 |
2-Aminobutanoic acid
CAS:2835-81-6 |
|
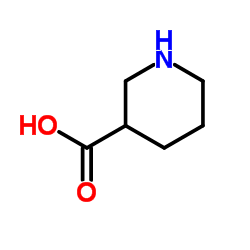 |
Nipecotic acid
CAS:498-95-3 |
|
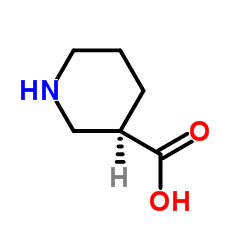 |
(R)-(-)-Nipecotic acid
CAS:25137-00-2 |
|
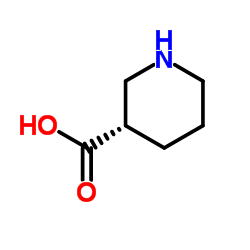 |
(S)-(+)-Nipecotic acid
CAS:59045-82-8 |
|
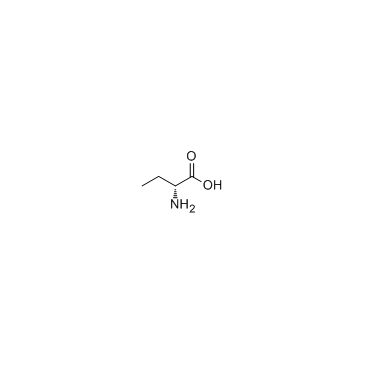 |
H-D-Abu-OH
CAS:2623-91-8 |