| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
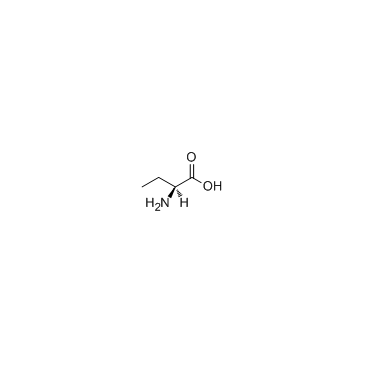 |
L-2-氨基丁酸
CAS:1492-24-6 |
|
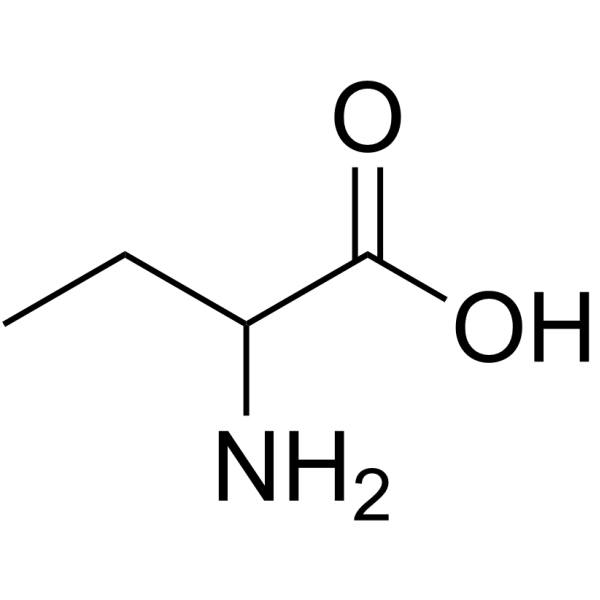 |
DL-2-氨基丁酸
CAS:2835-81-6 |
|
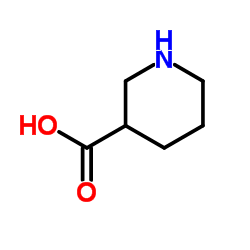 |
3-哌啶甲酸
CAS:498-95-3 |
|
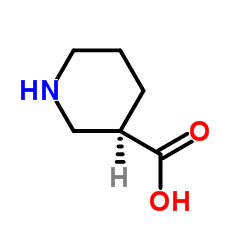 |
(R)-(-)-3-哌啶甲酸
CAS:25137-00-2 |
|
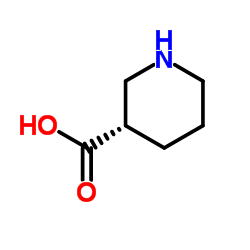 |
(S)-(+)-3-哌啶甲酸
CAS:59045-82-8 |
|
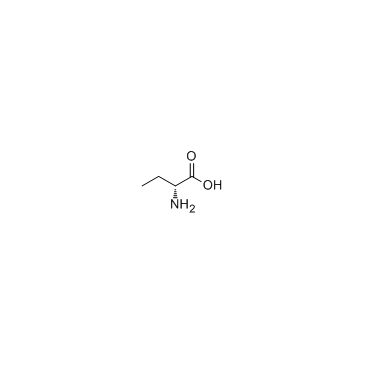 |
D-氨基丁酸
CAS:2623-91-8 |