| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Native Bovine Alkaline Phosphatase
CAS:9001-78-9 |
|
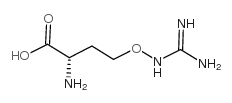 |
(L)-Canavanine
CAS:543-38-4 |