| Structure | Name/CAS No. | Articles |
|---|---|---|
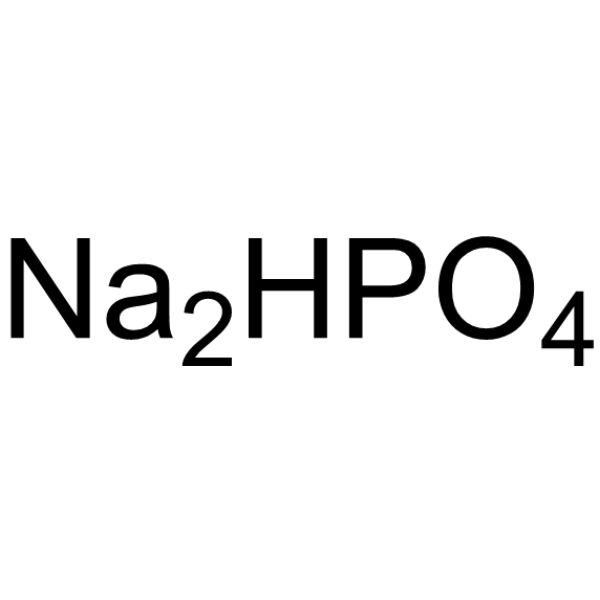 |
Disodium hydrogenorthophosphate
CAS:7558-79-4 |
|
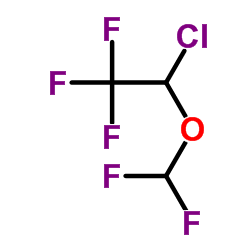 |
Isoflurane
CAS:26675-46-7 |
|
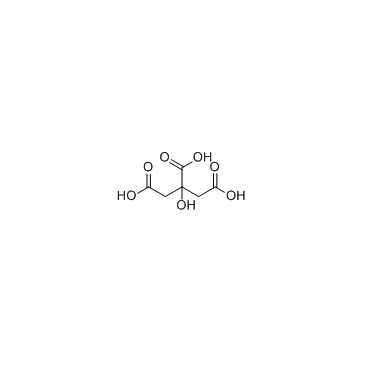 |
Citric Acid
CAS:77-92-9 |
|
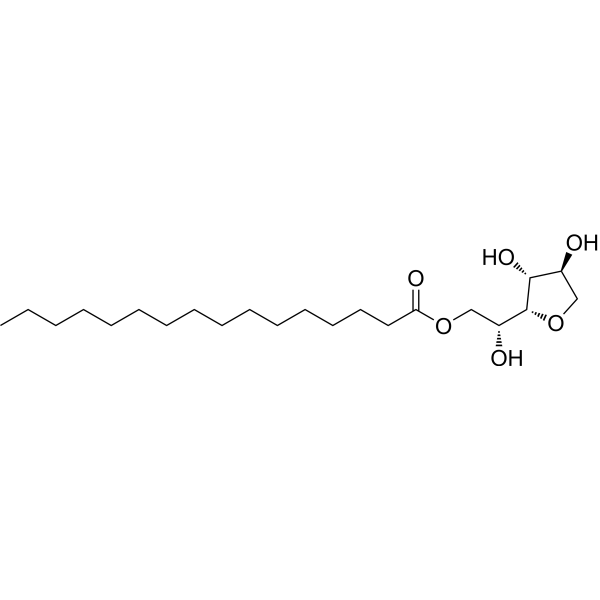 |
Span 40
CAS:26266-57-9 |
|
 |
Tricaprilin
CAS:538-23-8 |
|
 |
Acrylic acid
CAS:79-10-7 |
|
 |
UNII:TF4710DNP9
CAS:5094-24-6 |