| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Methanol
CAS:67-56-1 |
|
 |
Salicylic acid
CAS:69-72-7 |
|
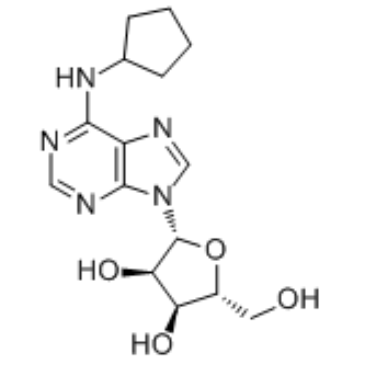 |
N6-Cyclopentyladenosine
CAS:41552-82-3 |
|
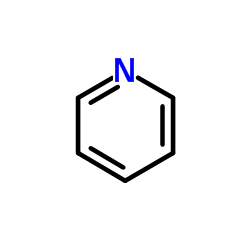 |
Pyridine
CAS:110-86-1 |
|
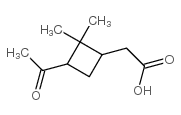 |
cis-Pinonic acid
CAS:61826-55-9 |
|
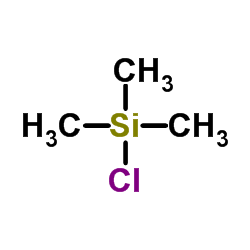 |
Chlorotrimethylsilane
CAS:75-77-4 |