| Structure | Name/CAS No. | Articles |
|---|---|---|
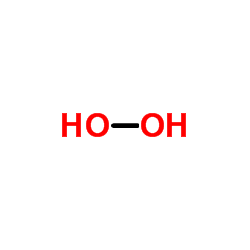 |
Hydrogen peroxide
CAS:7722-84-1 |
|
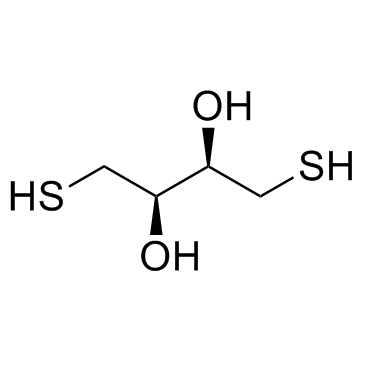 |
DL-Dithiothreitol
CAS:3483-12-3 |
|
 |
NAD+
CAS:53-84-9 |
|
 |
Sodium cyanide
CAS:143-33-9 |