| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Pyrogallol
CAS:87-66-1 |
|
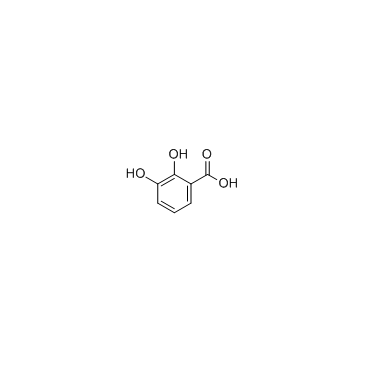 |
2,3-Dihydroxybenzoic acid
CAS:303-38-8 |
|
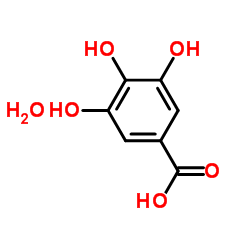 |
Gallic acid hydrate
CAS:5995-86-8 |
|
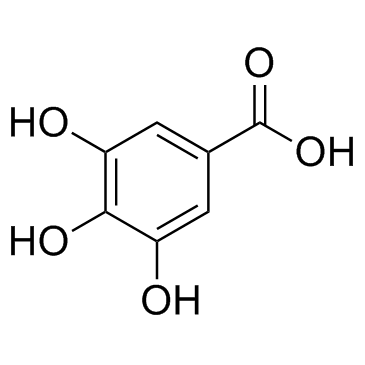 |
Gallic acid
CAS:149-91-7 |
|
 |
Shikimic acid
CAS:138-59-0 |
|
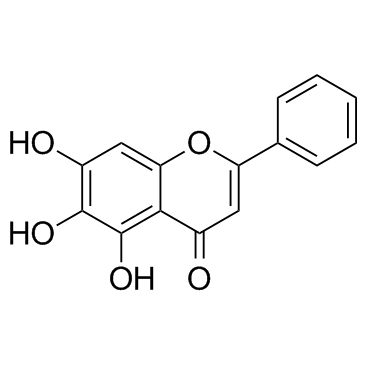 |
Baicalein
CAS:491-67-8 |
|
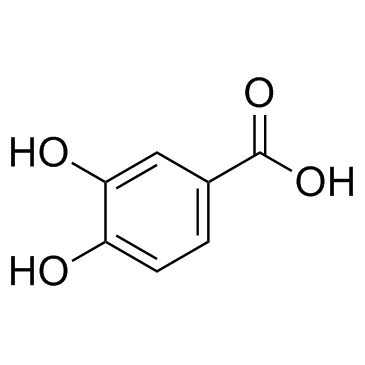 |
protocatechuic acid
CAS:99-50-3 |
|
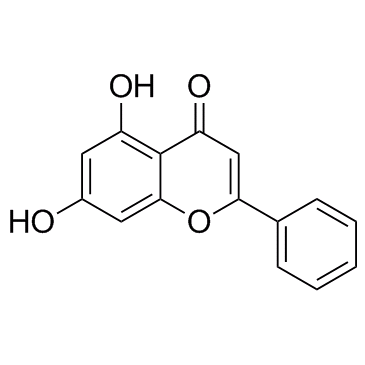 |
Chrysin
CAS:480-40-0 |