| Structure | Name/CAS No. | Articles |
|---|---|---|
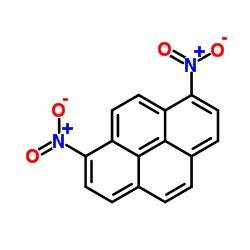 |
1,8-DINITROPYRENE
CAS:42397-65-9 |
|
 |
1,6-DINITROPYRENE
CAS:42397-64-8 |
|
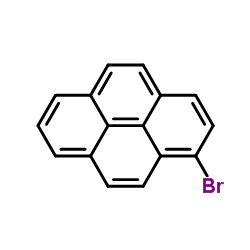 |
1-Bromopyrene
CAS:1714-29-0 |