| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
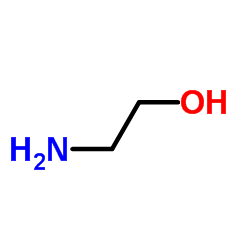 |
2-Aminoethanol
CAS:141-43-5 |
|
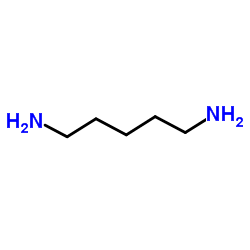 |
Cadaverine
CAS:462-94-2 |
|
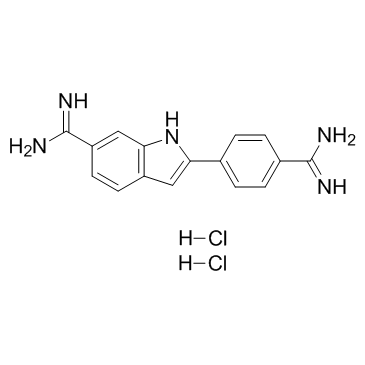 |
4',6-Diamidino-2-phenylindole dihydrochloride
CAS:28718-90-3 |
|
 |
Aucubin
CAS:479-98-1 |
|
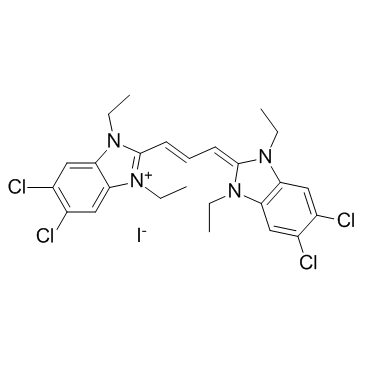 |
JC-1
CAS:3520-43-2 |
|
 |
DiSBAC2(5)
CAS:70363-83-6 |