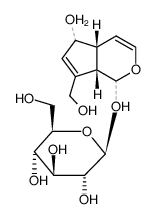
Aucubin

Aucubin structure
|
Common Name | Aucubin | ||
|---|---|---|---|---|
| CAS Number | 479-98-1 | Molecular Weight | 346.330 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 669.0±55.0 °C at 760 mmHg | |
| Molecular Formula | C15H22O9 | Melting Point | 180 - 184ºC | |
| MSDS | Chinese USA | Flash Point | 358.4±31.5 °C | |
Use of AucubinAucubin is an iridoid glycoside with a wide range of biological activities, including anti-inflammatory, anti-microbial, anti-algesic as well as anti-tumor activities.IC50 value:Target:In vitro: Aucubin promotes neuronal differentiation and neurite outgrowth in neural stem cells cultured primarily from the rat embryonic hippocampus [1]. Aucubin significantly reversed the elevated gene and protein expression of MMP-3, MMP-9, MMP-13, iNOS, COX-2 and the production of NO induced by IL-1β challenge in rat chondrocytes [2]. In vivo: |
| Name | Aucubin |
|---|---|
| Synonym | More Synonyms |
| Description | Aucubin is an iridoid glycoside with a wide range of biological activities, including anti-inflammatory, anti-microbial, anti-algesic as well as anti-tumor activities.IC50 value:Target:In vitro: Aucubin promotes neuronal differentiation and neurite outgrowth in neural stem cells cultured primarily from the rat embryonic hippocampus [1]. Aucubin significantly reversed the elevated gene and protein expression of MMP-3, MMP-9, MMP-13, iNOS, COX-2 and the production of NO induced by IL-1β challenge in rat chondrocytes [2]. In vivo: |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 669.0±55.0 °C at 760 mmHg |
| Melting Point | 180 - 184ºC |
| Molecular Formula | C15H22O9 |
| Molecular Weight | 346.330 |
| Flash Point | 358.4±31.5 °C |
| Exact Mass | 346.126373 |
| PSA | 149.07000 |
| LogP | -3.17 |
| Vapour Pressure | 0.0±4.6 mmHg at 25°C |
| Index of Refraction | 1.660 |
| Storage condition | 2-8°C |
|
~0% 
Aucubin CAS#:479-98-1 |
| Literature: Cachet, Xavier; Deguin, Brigitte; Koch, Michel; Makhlouf, Kader; Tillequin, Francois Journal of Natural Products, 1999 , vol. 62, # 2 p. 400 - 402 |
|
~% 
Aucubin CAS#:479-98-1 |
| Literature: Bianco; Passacantilli; Nicoletti; Alves de Lima Tetrahedron, 1982 , vol. 38, # 3 p. 359 - 362 |
|
~% 
Aucubin CAS#:479-98-1 |
| Literature: Bianco; Passacantilli; Nicoletti; Alves de Lima Tetrahedron, 1982 , vol. 38, # 3 p. 359 - 362 |
|
~% 
Aucubin CAS#:479-98-1 |
| Literature: Bianco; Passacantilli; Nicoletti; Alves de Lima Tetrahedron, 1982 , vol. 38, # 3 p. 359 - 362 |
|
~% 
Aucubin CAS#:479-98-1 |
| Literature: Weinges, Klaus; Ziegler, Hans Juergen Liebigs Annalen der Chemie, 1990 , # 7 p. 715 - 717 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Binding properties of antimicrobial agents to lipid membranes using surface plasmon resonance.
Biol. Pharm. Bull. 37(8) , 1383-9, (2014) In the present study, we examined the interaction of antimicrobial agents with four model lipid membranes that mimicked mammalian cell membranes and Gram-positive and -negative bacterial membranes and... |
|
|
Positive cooperativity between acceptor and donor sites of the peptidoglycan glycosyltransferase.
Biochem. Pharmacol. 93(2) , 141-50, (2015) The glycosyltransferases of family 51 (GT51) catalyze the polymerization of lipid II to form linear glycan chains, which, after cross linking by the transpeptidases, form the net-like peptidoglycan ma... |
|
|
Investigation of the interactions between the EphB2 receptor and SNEW peptide variants.
Growth Factors 32(6) , 236-46, (2014) EphB2 interacts with cell surface-bound ephrin ligands to relay bidirectional signals. Overexpression of the EphB2 receptor protein has been linked to different types of cancer. The SNEW (SNEWIQPRLPQH... |
| Rhimanthin |
| EINECS 207-540-8 |
| Aucubin |
| Rhinanthin |
| (2S,3R,4S,5S,6R)-2-{[(1S,4aR,5S,7aS)-5-Hydroxy-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-1-yl]oxy}-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol |
| Rhimantin |
| (1S,4aR,5S,7aS)-5-Hydroxy-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-1-yl β-D-glucopyranoside |
| β-D-Glucopyranoside, (1S,4aR,5S,7aS)-1,4a,5,7a-tetrahydro-5-hydroxy-7-(hydroxymethyl)cyclopenta[c]pyran-1-yl |
| AUCUBOSIDE |
| [1S-(1a,4aa,5a,7a)]-1,4a,5,7a-Tetrahydro-5-hydroxy-7-(hydroxymethyl)cyclopenta[c]pyran-1-yl-b-D-glucopyranoside |
| MFCD00136026 |
| (2S,3R,4S,5S,6R)-2-{[(1S,4aR,5S,7aS)-5-Hydroxy-7-(hydroxyméthyl)-1,4a,5,7a-tétrahydrocyclopenta[c]pyran-1-yl]oxy}-6-(hydroxyméthyl)tétrahydro-2H-pyran-3,4,5-triol |
| (2S,3R,4S,5S,6R)-2-{[(1S,4aR,5S,7aS)-5-Hydroxy-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyr-1-yl]oxy}-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol |
| (1S,4aR,5S,7aS)-5-Hydroxy-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyr-1-yl-β-D-glucopyranoside |
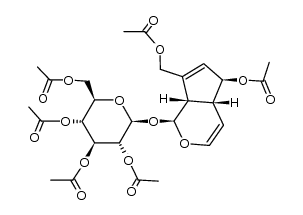

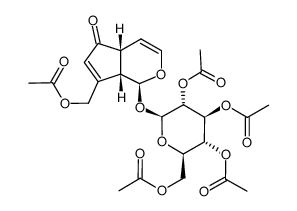
![(2R,3R,4S,5R,6S)-2-(acetoxymethyl)-6-(((1S,4aR,5S,7aS)-7-(acetoxymethyl)-5-hydroxy-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-1-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate structure](https://image.chemsrc.com/caspic/334/82504-04-9.png)


