| Structure | Name/CAS No. | Articles |
|---|---|---|
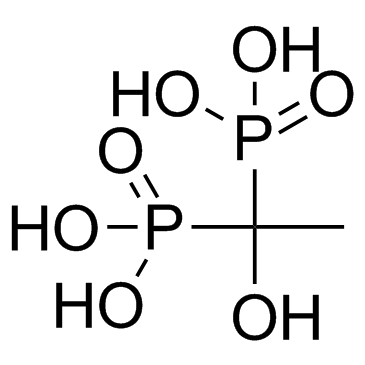 |
Etidronic acid
CAS:2809-21-4 |
|
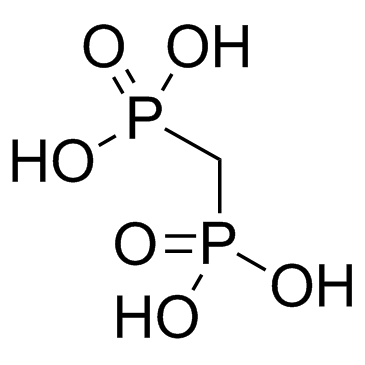 |
Medronic Acid
CAS:1984-15-2 |
|
 |
IMINO-BIS(METHYLPHOSPHONIC ACID)
CAS:17261-34-6 |
|
 |
Ampicillin
CAS:69-53-4 |
|
 |
IMIDODIPHOSPHATE SODIUM SALT
CAS:26039-10-1 |
|
 |
Aminotrimethylene phosphonic acid
CAS:6419-19-8 |