| Structure | Name/CAS No. | Articles |
|---|---|---|
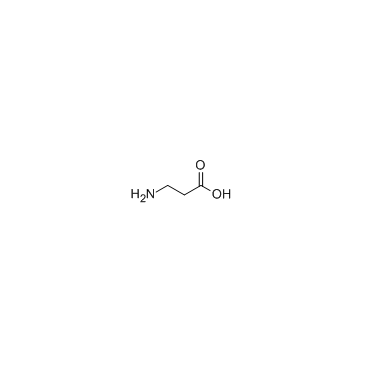 |
beta-Alanine
CAS:107-95-9 |
|
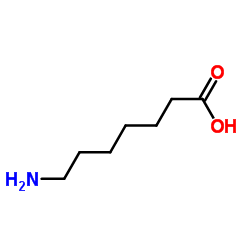 |
7-Aminoheptanoic acid
CAS:929-17-9 |
|
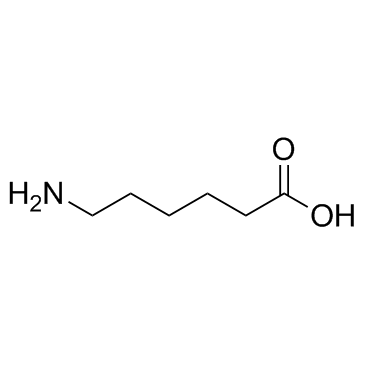 |
6-Aminocaproic acid
CAS:60-32-2 |
|
 |
4-Aminobutanoic acid
CAS:56-12-2 |
|
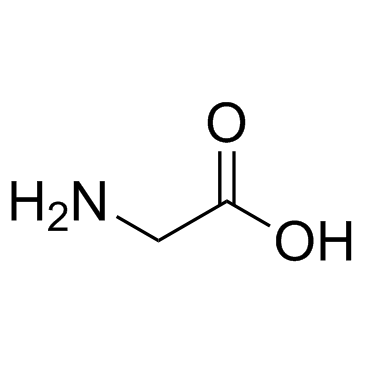 |
Glycine
CAS:56-40-6 |
|
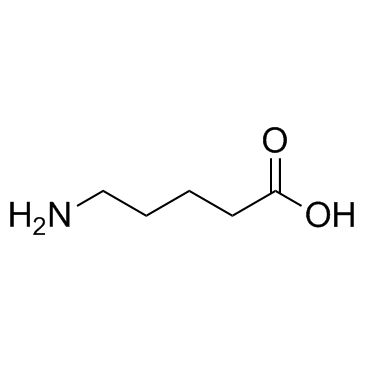 |
5-Aminovaleric acid
CAS:660-88-8 |
|
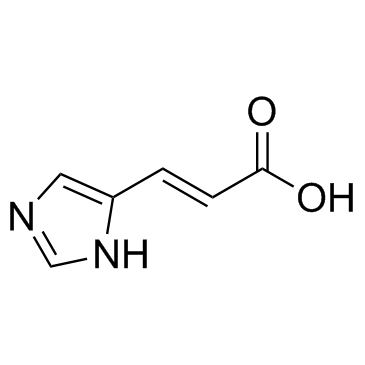 |
Urocanic acid
CAS:104-98-3 |