| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Glycerol
CAS:56-81-5 |
|
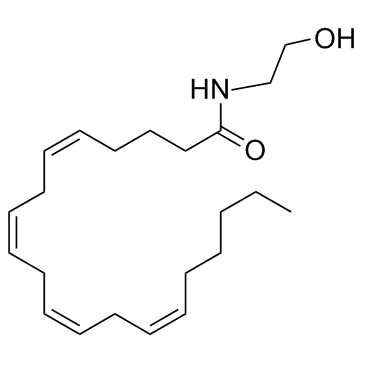 |
Anandamide
CAS:94421-68-8 |
|
 |
2-arachidonoylglycerol
CAS:53847-30-6 |